Multiple myeloma drugs enhance patient outcomes and mitigate the economic burden on healthcare systems and society. By effectively managing the disease through pharmaceutical treatments, these drugs improve patient well-being, reduce hospitalization rates, and extend life expectancy. The comprehensive healthcare approach, encompassing medical interventions and supportive care alongside drug therapy, aids in maintaining patients' health and quality of life. The economic impact is significant as successful treatment reduces the need for extensive healthcare resources, minimizing direct and indirect costs associated with prolonged hospital stays and supportive care measures
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-multiple-myeloma-drugs-market
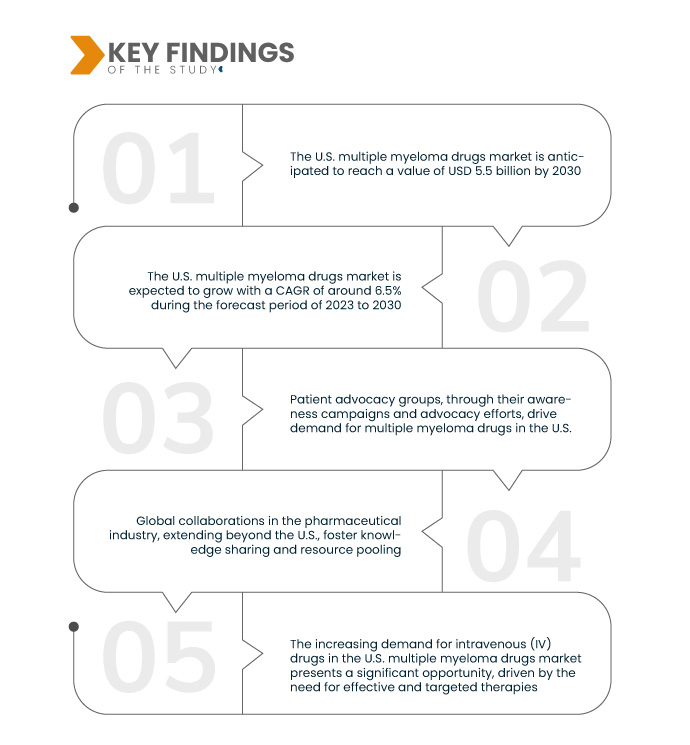
Data Bridge Market Research analyses the U.S. Multiple Myeloma Drugs Market, which was USD 1.79 billion in 2022, is expected to reach up to USD 5.5 billion by 2030, and is expected to undergo a CAGR of 15% during the forecast period of 2023 to 2030. Investments in precision medicine and biomarker research drive advancements in identifying precise molecular targets linked to multiple myeloma. This fosters the development of targeted therapies, enhancing treatment effectiveness and fostering personalized approaches.
Key Findings of the Study
Rising incidence of multiple myeloma due to an aging population is expected to drive the market's growth rate
The aging population in the U.S., coupled with other demographic factors, is a significant driver for the U.S. multiple myeloma drugs market. As individuals age, the incidence of multiple myeloma tends to rise. This demographic shift has resulted in an increased prevalence of the disease. There is a growing demand for drugs to manage and treat multiple myeloma within this expanding demographic effectively. The pharmaceutical industry responds to this demand by developing and providing innovative therapies, creating a substantial market opportunity driven by the healthcare needs of an aging population susceptible to multiple myeloma.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Disease (Smouldering (Indolent) Multiple Myeloma, Active (Symptomatic) Multiple Myeloma, Solitary Plasmacytoma of the Bone, Extrameduallry Plasmacytoma, Light Chain Myeloma, Non-Secretory Myeloma and Rare Types of Myeloma), Therapy (Immunotherapy, Chemotherapy, Corticosteroids, Others), Mechanism of Action (Proteasome Inhibitor, Monoclonal Antibodies, Immunomodulatory Agents, Histone Deacetylase Inhibitors, Others), Brands (Darzalex, Ninlaro, Revlimod, Velcade, Empliciti, Venclexta, Others), Drug Type (Branded, Generic), Route of Administration (Intravenous, Oral, Subcutaneous, Intramuscular, Intra-Arterial, Others), End User (Hospitals, Specialty Clinics, Academic and Research Institutes and Others), Distribution Channel (Retail Pharmacy and Drug Store, Hospital Pharmacy, Online Pharmacy, Others)
|
|
Market Players Covered
|
Sanofi (France), Pfizer Inc. (U.S.), GSK plc (U.K.), Novartis AG (Switzerland), AbbVie Inc. (U.S.), C.R. Bard (U.S.), Straub Medical AG (U.S.), FlowJo LLC (U.S.), NeoGenomics Laboratories (U.S.), Helena Laboratories Corporation (U.S.), Bracco (Italy), ZYTOVISION GmbH (Germany), Adaptive Biotechnologies. (U.S.), BioVendor RandD (U.S.), TriVitron Healthcare (India), Eurofins Discovery (France), Quest Drugs Incorporated (U.S.), Takeda Pharmaceutical Company Limited (Japan)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The U.S. multiple myeloma drugs market is segmented on the basis of disease, therapy, mechanism of action, brands, drug type, route of administration, end user, and distribution channel.
- On the basis of disease, the U.S. multiple myeloma drugs market is segmented into smouldering (indolent) multiple myeloma, active (symptomatic) multiple myeloma, solitary plasmacytoma of the bone, extrameduallry plasmacytoma, light chain myeloma, non-secretory myeloma and rare types of myeloma
- On the basis of therapy, the U.S. multiple myeloma drugs market is segmented into immunotherapy, chemotherapy, corticosteroids, and others
- On the basis of mechanism of action, the U.S. multiple myeloma drugs market is segmented into proteasome inhibitor, monoclonal antibodies, immunomodulatory agents, histone deacetylase inhibitors, and others
- On the basis of brands, the U.S. multiple myeloma drugs market is segmented into darzalex, ninlaro, revlimod, velcade, empliciti, venclexta, and others
- On the basis of drug type, the U.S. multiple myeloma drugs market is segmented into branded, and generic
- On the basis of route of administration, the U.S. multiple myeloma drugs market is segmented into intravenous, oral, subcutaneous, intramuscular, intra-arterial, and others
- On the basis of end-user, the U.S. multiple myeloma drugs market is segmented into hospitals, specialty clinics, academic and research institutes and others
- On the basis of distribution channel, the U.S. multiple myeloma drugs market is segmented into retail pharmacy and drug store, hospital pharmacy, online pharmacy, and others
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. multiple myeloma drugs market players in U.S. multiple myeloma drugs market are Sanofi (France), Pfizer Inc. (U.S.), GSK plc (U.K.), Novartis AG (Switzerland), AbbVie Inc. (U.S.), C.R. Bard (U.S.), Straub Medical AG (U.S.), FlowJo LLC (U.S.), NeoGenomics Laboratories (U.S.), Helena Laboratories Corporation (U.S.)
Market Developments
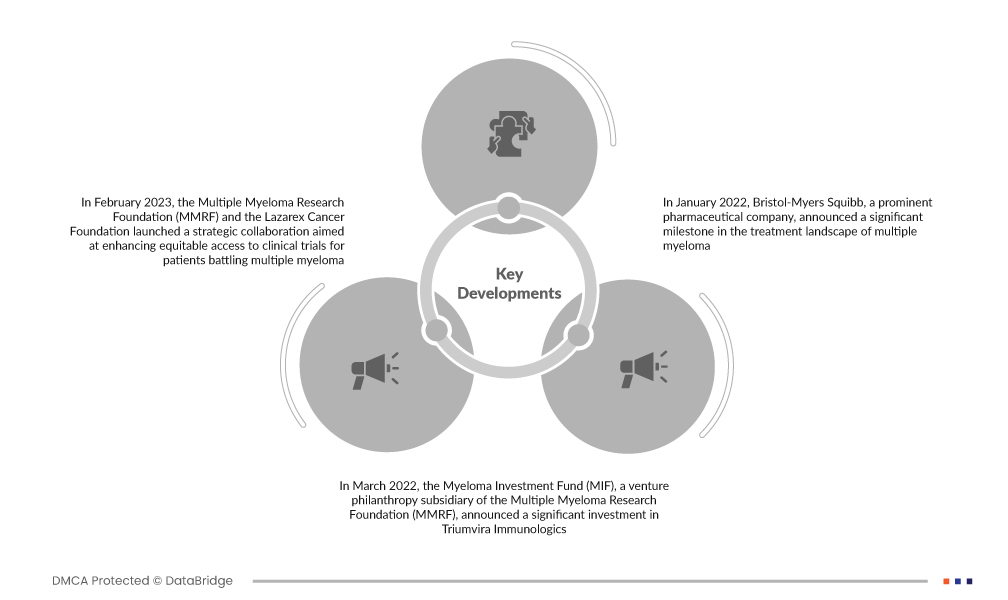
- In February 2023, the Multiple Myeloma Research Foundation (MMRF) and the Lazarex Cancer Foundation announced a strategic collaboration to enhance equitable access to clinical trials for patients battling multiple myeloma. The Lazarex Cancer Foundation, a non-profit organization dedicated to improving patient access to cancer clinical trials, joined forces with MMRF to address the need for increased inclusivity in clinical trials. This collaboration is anticipated to have a profound impact on the demand for multiple myeloma drugs and therapies, thereby catalyzing market growth
- In March 2022, the Myeloma Investment Fund (MIF), a venture philanthropy subsidiary of the Multiple Myeloma Research Foundation (MMRF), announced a significant investment in Triumvira Immunologics. Triumvira, a clinical-stage biotech company based in Austin, TX, specializes in developing novel, targeted autologous and allogeneic T cell immunotherapies. These innovative therapies leverage T cells' natural biology to combat solid tumors and blood cancers. The strategic investment by MIF underscores the growing interest and financial support in advancing groundbreaking immunotherapies for the treatment of multiple myeloma
- In January 2022, Bristol-Myers Squibb, a prominent pharmaceutical company, announced a significant milestone in the treatment landscape of multiple myeloma. Japan’s Ministry of Health, Labour and Welfare approved Abecma (idecabtagene vicleucel), a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell immunotherapy. This approval specifically pertains to the treatment of adult patients grappling with relapsed or refractory (R/R) multiple myeloma. The regulatory green light for Abecma underscores the continued advancement of innovative therapies in addressing the complex challenges associated with multiple myeloma
For more detailed information about the U.S. multiple myeloma drugs market report, click here – https://www.databridgemarketresearch.com/reports/us-multiple-myeloma-drugs-market