Asia Pacific Mycoplasma Testing In Clinical Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
52.52 Million
USD
98.66 Million
2022
2030
USD
52.52 Million
USD
98.66 Million
2022
2030
| 2023 –2030 | |
| USD 52.52 Million | |
| USD 98.66 Million | |
|
|
|
Asia-Pacific Mycoplasma Testing in Clinical Market, By Products (Kits and Reagents, Instruments, Services), Technique (Microbial Culture Techniques/Direct Assay, Polymerase Chain Reaction, ELISA, DNA Staining/Indirect Assay, Enzymatic Methods), Application (Cell Line Testing, Virus Testing, End of Production Cells Testing, Others), Disease Area (Respiratory, Urogenital, Gastrointestinal, Musculoskeletal, Cardiovascular, Others), End User (Diagnostic Laboratories, Hospitals) – Industry Trends and Forecast to 2030.
Asia-Pacific Mycoplasma Testing in Clinical Market Analysis and Size
The CDC predicts that 152,657 people passed away in 2021 as a result of chronic lower respiratory illnesses. The market for mycoplasma testing in clinical settings is expanding due to the rising number of deaths from respiratory illnesses, pulmonary diseases and tuberculosis, and these numbers are predicted to continue to rise in the upcoming years. Presently, clinical and public health laboratories frequently use molecular test techniques. The U.S. Food and Drug Administration (FDA) has approved a number of commercially available molecular test kits to identify M. pneumoniae. The majority of these kits are utilized for the detection of M. pneumoniae as well as other respiratory infections. Local or state public health laboratories can offer diagnostic help or send samples to CDC when additional or specialized testing is required.
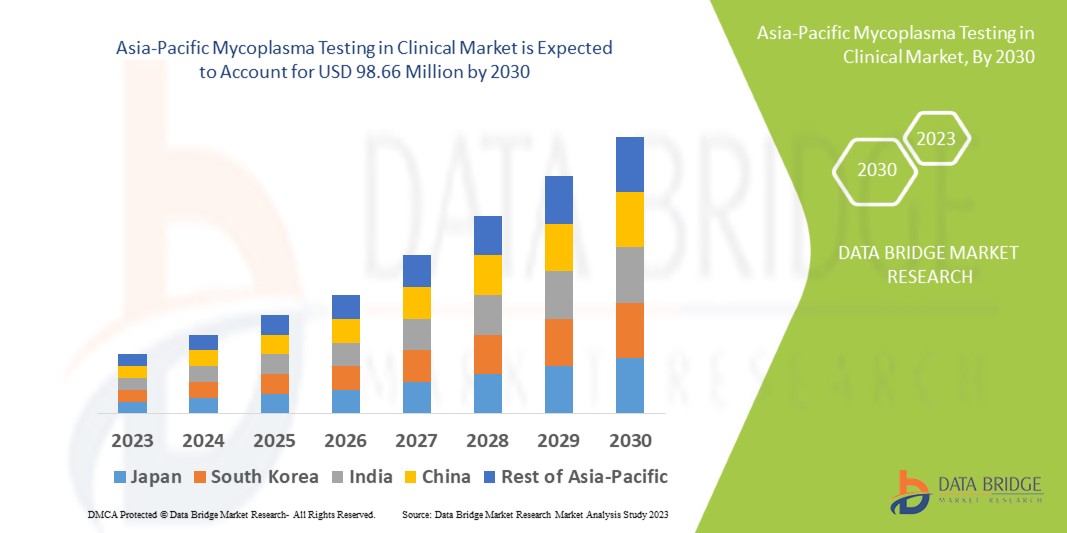
Data Bridge Market Research analyses that the mycoplasma testing in clinical market which was USD 52.52 million in 2022, is expected to reach USD 98.66 million by 2030, at a CAGR of 8.2% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Asia-Pacific Mycoplasma Testing in Clinical Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Products (Kits and Reagents, Instruments, Services), Technique (Microbial Culture Techniques/Direct Assay, Polymerase Chain Reaction, ELISA, DNA Staining/Indirect Assay, Enzymatic Methods), Application (Cell Line Testing, Virus Testing, End of Production Cells Testing, Others), Disease Area (Respiratory, Urogenital, Gastrointestinal, Musculoskeletal, Cardiovascular, Others), End User (Diagnostic Laboratories, Hospitals) |
|
Countries Covered |
China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC) |
|
Market Players Covered |
AB ANALITICA s.r.l. (Italy), BIOMÉRIEUX (France), ELITechGroup (France), Liofilchem S.r.l. (Italy), Agilent Technologies, Inc. (U.S.), PromoCell GmbH (Germany), F. Hoffmann-La Roche Ltd (Switzerland), OSANG Healthcare (South Korea), Sacace Biotechnologies Srl (Italy), Lonza (Switzerland), Merck KGaA (Germany), Seegene Inc. (South Korea), Clongen Laboratories, LLC (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Charles River Laboratories (U.S.), Bionique Testing Laboratories LLC (U.S.) and ZEAKON Diagnostics (India) |
|
Market Opportunities |
|
Market Definition
Mycoplasma is a form of bacterial infection that can be found by using a mycoplasma test. There are various mycoplasma infections, testing can be carried out in a number of methods depending on which one is suspected. M. pneumoniae, which causes upper respiratory infections and can be detected with a blood sample, is mycoplasma's most prevalent. A vaginal swab, joint fluid, bodily fluids, tissue samples and other sample types may need to be collected for further mycoplasma testing such as sputum.
Asia-Pacific Mycoplasma Testing in Clinical Market Dynamics
Drivers
- Growing R&D investments
The adoption of highly effective mycoplasma testing technologies will certainly be encouraged by rising research expenditures and rising R&D investments by major corporations. For instance, Bristol-Myers Squibb started spending money on creating experimental drugs to help clinical trials. Furthermore, it is essential to carry out safety testing to make sure that a product meets the requirements for efficacy, safety and general public health. This is because of the emergence of biosimilars and the lucrative R&D investment that followed. In December 2020, Merck KGaA increased the size of its American manufacturing footprint by investing more than USD 45.6 million (EUR 40 million) in its Massachusetts and New Hampshire production sites. The facilities were created to increase the company's capability for production while producing a range of biopharmaceutical manufacturing products. These are the factors which further propel the market growth.
- Growing concerns over cell culture contamination
Contamination is the most frequent issue in cell culture laboratories. Chemical and biological contamination are the two categories into which cell culture contaminations are divided. Serum, water, endotoxins and impurities are a few instances of chemical pollutants. Testing for mycoplasma is one of the procedures used to find contamination.
Opportunities
- Globalization of clinical trials
ELISA technology is predicted to achieve a sizable market share throughout the forecast period because it allows for simple detection using labeled probes or antibodies for mycoplasma detection. In some cases, PCR and ELISA tests are combined to create PCR-ELISA, a photometric enzyme-based immunoassay that makes it easier to find mycoplasma DNA that PCRPCR has amplified has amplified in samples. The segment growth will be fuelled by factors such as functional advantages, cost effectiveness, and the capacity to identify a wide variety of strains during the forecast period.
Restraints/Challenges
- High cost of instruments
The mycoplasma testing in clinical market expansion is anticipated to be restrained by the high cost of equipment and the time-consuming and tedious detection method, during the forecast period. One of the most frequent causes of both paediatric and adult community-acquired pneumonia is Mycoplasma pneumoniae.
This mycoplasma testing in clinical market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the mycoplasma testing in clinical market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
• In 2020, Liofilchem S.r.l. Introduced the enhanced AF Genital System, VTM, Lockable contact plates with microbiological testing. The company's revenue increased because to this product upgrade.
• In 2020, BioMérieux SA announced the introduction of the biofire mycoplasma test for the detection of mycoplasma in biopharmaceutical products. This strategy helped the business increase the market shares of its product in the biotherapeutics sector.
COVID-19 Impact on the Mycoplasma Testing in Clinical Market
The COVID-19 outbreak has had a detrimental impact on all sectors of the economy and is still having a negative effect. When compared to other industries, the biopharmaceutical industry and adjacent markets have not been considerably affected. A study released in May 2022 found a connection between the quantity of mycoplasma tests and COVID-19-related non-pharmaceutical interventions (NPIs). The incidence of M. pneumonia following the adoption of COVID-19 NPIs was examined in this study. This study revealed a decline in M. pneumoniae detection throughout the epidemic. This decrease was noticed between March 2020 and March 2021. This decrease is mostly attributable to the limited spread of M. pneumonia, which has led to a sharp decline in M. pneumoniae infections worldwide.
Asia-Pacific Mycoplasma Testing in Clinical Market Scope
The mycoplasma testing in clinical market is segmented on the basis of products, technique, application, disease area and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Products
- Kits and Reagents
- PCR Assays
- Nucleic Acid Detection Kits
- Stains
- Elimination Kits
- Standards and Controls
- Others
- Instruments
- Services
Technique
- Microbial Culture Techniques/Direct Assay
- Polymerase Chain Reaction
- ELISA
- DNA Staining/Indirect Assay
- Enzymatic Methods
Application
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
Disease Area
- Respiratory
- Urogenital
- Gastrointestinal
- Musculoskeletal
- Cardiovascular
- Others
End User
- Diagnostic Laboratories
- Hospitals
Mycoplasma Testing in Clinical Market Regional Analysis/Insights
The mycoplasma testing in clinical market is analyzed and market size insights and trends are provided by country, products, technique, application disease area and end user as referenced above.
The countries covered in the mycoplasma testing in clinical market report are China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC).
Japan is leading the growth in the market due to rise in healthcare expenditure, growing research activities conducted by pharmaceutical and biotechnology companies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The mycoplasma testing in clinical market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for mycoplasma testing in clinical market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the mycoplasma testing in clinical market. The data is available for historic period 2011-2021.
Competitive Landscape and Mycoplasma Testing in Clinical Market Share Analysis
The mycoplasma testing in clinical market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to mycoplasma testing in clinical market.
Some of the major players operating in the mycoplasma testing in clinical market are:
- AB ANALITICA s.r.l. (Italy)
- BIOMÉRIEUX (France)
- ELITechGroup (France)
- Liofilchem S.r.l. (Italy)
- Agilent Technologies, Inc. (U.S.)
- PromoCell GmbH (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- OSANG Healthcare (South Korea)
- Sacace Biotechnologies Srl (Italy)
- Lonza (Switzerland)
- Merck KGaA (Germany)
- Seegene Inc. (South Korea)
- Clongen Laboratories, LLC (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Charles River Laboratories (U.S.)
- Bionique Testing Laboratories LLC (U.S.)
- ZEAKON Diagnostics (India)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.