Europe Postpartum Hemorrhage Treatment Devices Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
274.95 Million
USD
397.15 Million
2024
2032
USD
274.95 Million
USD
397.15 Million
2024
2032
| 2025 –2032 | |
| USD 274.95 Million | |
| USD 397.15 Million | |
|
|
|
|
Mercado de dispositivos para tratamento de hemorragia pós-parto na Europa, tipo (tamponamento com balão uterino, sistema de injeção pré-preenchido Uniject, vestimenta antichoque não pneumática, dispositivos de controle de hemorragia induzida por vácuo e outros), condição (hemorragia pós-parto grave (mais de 1000 ml), hemorragia pós-parto leve (500-1000 ml), hemorragia pós-parto maciça (2000 ml ou mais) e hemorragia pós-parto secundária), tipo de paciente (HPP primária e HPP secundária), usuário final (hospitais, maternidades, clínicas especializadas, ambientes de atendimento domiciliar e outros), canal de distribuição (licitação direta, vendas no varejo e outros) - Tendências do setor e previsão até 2032
Tamanho do mercado de dispositivos para tratamento de hemorragia pós-parto
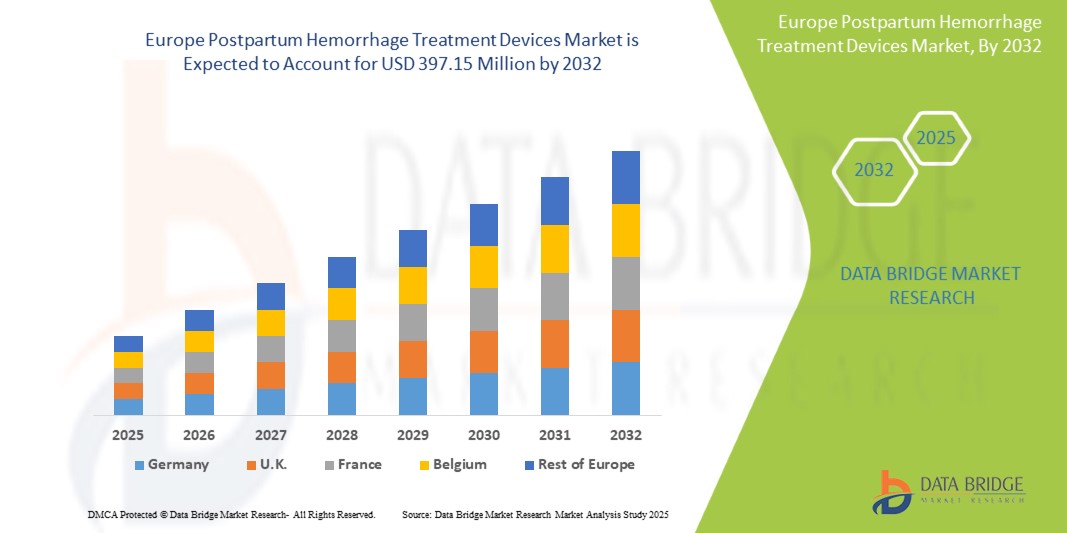
- O mercado europeu de dispositivos para tratamento de hemorragia pós-parto foi avaliado em US$ 274,95 milhões em 2024 e deverá atingir US$ 397,15 milhões até 2032.
- Durante o período previsto de 2025 a 2032, o mercado deverá crescer a uma CAGR de 4,7%, impulsionado principalmente pela crescente incidência de hemorragia pós-parto
- Os principais impulsionadores do mercado de dispositivos para tratamento de hemorragia pós-parto incluem a crescente incidência de hemorragia pós-parto, a conscientização crescente sobre o tratamento eficaz e os avanços na tecnologia
Análise de dispositivos para tratamento de hemorragia pós-parto
- A crescente incidência de hemorragia pós-parto, motivada por fatores como o aumento das taxas de cesáreas e complicações durante o parto, resultou numa crescente procura por dispositivos de tratamento eficazes.
- As inovações em tecnologia médica, incluindo novos dispositivos de tamponamento uterino, agentes hemostáticos e opções cirúrgicas minimamente invasivas, estão contribuindo para melhorias nos resultados dos pacientes e impulsionando o crescimento do mercado.
- Por exemplo, o mercado de dispositivos para tratamento de HPP varia geograficamente, com um crescimento significativo observado nas regiões em desenvolvimento devido ao aumento dos investimentos em saúde e ao foco na saúde materna.
- Portanto, o mercado de dispositivos para tratamento de HPP demonstra uma forte disparidade geográfica, com as regiões em desenvolvimento experimentando um crescimento significativo devido ao aumento dos investimentos em saúde e uma abordagem focada na melhoria da saúde materna.
Escopo do Relatório e Segmentação de Dispositivos para Tratamento de Hemorragia Pós-Parto
|
Atributos |
Principais insights de mercado sobre dispositivos para tratamento de hemorragia pós-parto |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análise de importação e exportação, visão geral da capacidade de produção, análise de consumo de produção, análise de tendência de preço, cenário de mudança climática, análise da cadeia de suprimentos, análise da cadeia de valor, visão geral de matéria-prima/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de dispositivos para tratamento de hemorragia pós-parto
“Aumento da adoção de dispositivos de tamponamento uterino com balão”
- Os dispositivos de tamponamento uterino com balão são projetados para controlar a HPP, fornecendo pressão direta na parede uterina, promovendo a hemostasia. Sua eficácia em ambientes clínicos tem sido amplamente documentada, levando a uma maior confiança entre os profissionais de saúde no uso desses dispositivos como intervenção de primeira linha para o tratamento da HPP, impulsionando assim sua adoção.
- Por exemplo, houve um esforço conjunto na comunidade de saúde para aprimorar o treinamento e a educação sobre o manejo da HPP. Isso inclui treinamento específico sobre o uso de técnicas de tamponamento uterino com balão. A maior conscientização entre os profissionais de saúde sobre a importância da intervenção imediata levou a uma maior aceitação e utilização desses dispositivos tanto em regiões desenvolvidas quanto em desenvolvimento.
- Os dispositivos de tamponamento uterino com balão estão sendo cada vez mais integrados aos protocolos abrangentes de cuidados maternos, especialmente em ambientes obstétricos de emergência
- Essa integração é apoiada por diretrizes de organizações como a OMS e diversas iniciativas de saúde materna, que promovem práticas baseadas em evidências para prevenir e tratar a HPP de forma eficaz. O alinhamento desses dispositivos com as diretrizes clínicas está facilitando sua ampla adoção em hospitais e maternidades.
Motoristas
“ Aumento da incidência de hemorragia pós-parto ”
- A crescente incidência de Hemorragia Pós-Parto (HPP) criou uma necessidade urgente de dispositivos de tratamento eficazes no mercado global. A maior conscientização sobre os problemas de saúde materna e a melhor identificação das populações em risco levaram a um aumento na demanda por soluções inovadoras.
- À medida que os sistemas de saúde priorizam a segurança materna, a adoção de dispositivos médicos avançados destinados a prevenir ou controlar a HPP está aumentando, incluindo dispositivos de compressão uterina e agentes hemostáticos.
Por exemplo,
- Em maio de 2020, segundo o NCBI, a Hemorragia Pós-Parto (HPP) é a principal causa de mortalidade materna em todo o mundo. Nos EUA, a HPP aumentou 26%. Esse aumento alarmante nos casos destaca a importância crucial de abordar a HPP e serve como catalisador para o aumento do investimento em soluções e tecnologias inovadoras de tratamento, visando prevenir e controlar essa condição potencialmente fatal.
- Em agosto de 2024, a Organização Mundial da Saúde declarou que a Hemorragia Pós-Parto (HPP), comumente definida como uma perda de sangue de 500 ml ou mais em 24 horas após o parto, é a principal causa de mortalidade materna em todo o mundo. Afeta milhões de mulheres todos os anos e é responsável por mais de 20% de todas as mortes maternas relatadas globalmente.
Oportunidades
“ Programas de treinamento e educação para o uso adequado de dispositivos de tratamento de HPP ”
- O desenvolvimento de programas abrangentes de treinamento e educação para o uso adequado de dispositivos de tratamento de HPP (hemorragia pós-parto) representa uma oportunidade valiosa para fortalecer os serviços de saúde materna.
- Estes programas podem ser adaptados para profissionais de saúde em vários níveis, desde agentes de saúde comunitários a parteiras qualificadas e pessoal hospitalar, garantindo que todo o pessoal envolvido nos cuidados maternos esteja equipado com o conhecimento e a experiência prática necessários.
Restrições/Desafios
“ Impacto ambiental e questões de descarte de dispositivos PPH de uso único ”
- Dispositivos descartáveis para hemorragia pós-parto (HPP), como tamponadores de balão uterino e sistemas de sucção, geram resíduos médicos significativos, apresentando desafios ambientais e de descarte, especialmente em locais com poucos recursos. Sem uma infraestrutura adequada de gerenciamento de resíduos, os dispositivos descartados podem se acumular em aterros sanitários ou lixões a céu aberto, liberando materiais nocivos, como plásticos e resíduos biológicos, no meio ambiente.
- O descarte inadequado também aumenta o risco de descarte ou reutilização de equipamentos contaminados em situações desesperadoras, aumentando o potencial de infecções como hepatite ou sepse entre pacientes e profissionais de saúde. Essa dupla ameaça – degradação ambiental e riscos à saúde pública – ressalta a necessidade de soluções sustentáveis de design e descarte no desenvolvimento de dispositivos de HPP.
Por exemplo,
- Em março de 2022, o MDPI destacou que o uso crescente de dispositivos descartáveis para hemorragia pós-parto contribui para o aumento do volume de resíduos hospitalares, muitos dos quais são perigosos e contribuem para riscos de infecção e poluição ambiental. O descarte inadequado e a crescente dependência de descartáveis, como seringas e cateteres, exigem atenção urgente à gestão sustentável de resíduos na área da saúde materna.
- Em dezembro de 2024, conforme a Centurial, embora os dispositivos descartáveis para tratamento de HPP aumentem a segurança e reduzam os riscos de infecção, seu uso generalizado levanta preocupações ambientais. O descarte inadequado contribui para o acúmulo de resíduos médicos e a poluição. Equilibrar a necessidade de instrumentos estéreis e descartáveis com a gestão sustentável de resíduos é essencial para minimizar os danos ambientais e, ao mesmo tempo, garantir um cuidado materno eficaz.
Escopo do mercado de dispositivos para tratamento de hemorragia pós-parto na Europa
O mercado europeu de dispositivos para tratamento de hemorragia pós-parto é categorizado em cinco segmentos notáveis que são baseados no tipo, condição, tipo de paciente, usuário final e canal de distribuição.
|
Segmentação |
Sub-segmentação |
|
Por tipo |
|
|
Por Condição |
|
|
Por tipo de paciente |
|
|
Por usuário final
|
|
|
Por canal de distribuição |
|
Análise regional do mercado europeu de dispositivos para tratamento de hemorragia pós-parto
“A Alemanha é o país dominante no mercado de dispositivos para tratamento de hemorragia pós-parto ”
- A Alemanha possui um sistema de saúde bem estabelecido com altos padrões de atendimento materno. As políticas proativas do governo e os investimentos substanciais em saúde materna contribuem para a ampla adoção de dispositivos avançados para o tratamento da HPP em todo o país.
- O país tem uma população significativa em risco de desenvolver condições associadas à HPP grave, como gestações múltiplas, miomas uterinos, anemia e síndrome HELLP. Esse risco elevado impulsiona a demanda por soluções eficazes para o tratamento da HPP, posicionando a Alemanha como um mercado líder na Europa.
- A Alemanha abriga grandes empresas no setor de dispositivos para tratamento de HPP, incluindo a Cook Medical e a Utah Medical Products. Essas empresas têm forte presença no mercado alemão, oferecendo uma gama de produtos inovadores, como tamponadores uterinos com balão, que têm alta demanda devido à sua facilidade de uso e eficácia.
A Alemanha deverá registrar o maior CAGR do mercado ”
- A Alemanha tem uma população significativa em risco de desenvolver condições associadas à HPP grave, como gestações múltiplas, miomas uterinos, anemia e síndrome HELLP. Esse risco elevado impulsiona a demanda por soluções eficazes para o tratamento da HPP, posicionando a Alemanha como um mercado líder na Europa.
- A Alemanha possui um sistema de saúde bem estabelecido com altos padrões de atendimento materno. As políticas proativas do governo e os investimentos substanciais em saúde materna contribuem para a ampla adoção de dispositivos avançados para o tratamento da HPP em todo o país.
Participação no mercado de dispositivos para tratamento de hemorragia pós-parto
O cenário competitivo do mercado fornece detalhes por concorrente. Os detalhes incluem visão geral da empresa, finanças da empresa, receita gerada, potencial de mercado, investimento em pesquisa e desenvolvimento, novas iniciativas de mercado, presença na Europa, locais e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento de produto, abrangência e amplitude do produto e domínio da aplicação. Os dados fornecidos acima referem-se apenas ao foco das empresas em relação ao mercado.
Os principais líderes de mercado que operam no mercado são:
- BD (EUA)
- Grupo de empresas Organon (Holanda)
- Laborie (EUA)
- Cooper Companies (EUA)
- Belmont Medical Technologies (EUA)
- Utah Medical Products, Inc. (EUA)
- Angiplast Private Limited (Índia)
- Krishco Medical Products Pvt. Ltd. (Índia)
- 3º Design de Pedra (EUA)
- Advin Health Care (EUA)
- Coagulant Therapeutics Corporation (EUA)
- Sterimed Group (EUA)
- RevMedx (EUA)
- Maternova Inc (EUA)
- Sinapi Biomedical (EUA)
Últimos desenvolvimentos em dispositivos para tratamento de hemorragia pós-parto
- Em abril de 2025, a Organon adquiriu os direitos nos EUA do TOFIDENCE, um biossimilar de tocilizumabe ao ACTEMRA, da Biogen. Isso fortalece o portfólio de biossimilares da Organon em imunologia, expandindo as opções de tratamento para artrite e COVID-19. O TOFIDENCE, lançado em maio de 2024, trata diversas condições inflamatórias e impulsiona o crescimento dos negócios de biossimilares da Organon, com significativo potencial de mercado.
- Em novembro de 2023, a CooperCompanies adquiriu ativos selecionados da Cook Medical por US$ 300 milhões, aprimorando seu portfólio de saúde feminina e cirurgia sob a CooperSurgical. O negócio inclui produtos como o balão Bakri e os monitores Doppler. Com previsão de aumento da receita e dos lucros em 2024, a aquisição fortalece a posição da Cooper na Europa em fertilidade e saúde ginecológica.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 MARKET END USER COVERAGE GRID
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACROECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.4 COST ANALYSIS BREAKDOWN
4.5 TECHNOLOGY ROADMAP
4.6 VALUE CHAIN ANALYSIS
4.7 OPPORTUNITY MAP ANALYSIS
4.8 HEALTHCARE ECONOMY
4.9 REIMBURSEMENT FRAMEWORK
4.1 TARIFFS AND ITS IMPACT ON THE MARKET
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 EUROPE VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE
6.1.2 ONGOING TECHNOLOGICAL ADVANCEMENTS FOR POSTPARTUM HEMORRHAGE TREATMENTS
6.1.3 RISING BIRTH RATES ASSOCIATED WITH AN INCREASE IN THE NUMBER OF POSTPARTUM HEMORRHAGE
6.1.4 REGULATORY SUPPORT AND APPROVALS ASSOCIATED WITH THE TREATMENT DEVICES
6.2 RESTRAINTS
6.2.1 SIDE EFFECTS ASSOCIATED WITH THE POSTPARTUM HEMORRHAGE TREATMENT
6.2.2 LIMITED RESEARCH AND DEVELOPMENT FOR PPH TREATMENT
6.3 OPPORTUNITIES
6.3.1 TRAINING AND EDUCATIONAL PROGRAMS FOR PROPER USE OF PPH TREATMENT DEVICES
6.3.2 SUPPORT FROM GOVERNMENTAL AND NON-GOVERNMENTAL ORGANIZATIONS IN PPH DEVICE ADOPTION
6.3.3 TELEMEDICINE INTEGRATION TO ENHANCE POSTPARTUM HEMORRHAGE DEVICE USE
6.4 CHALLENGES
6.4.1 ENVIRONMENTAL IMPACT AND DISPOSAL ISSUES OF SINGLE-USE PPH DEVICES
6.4.2 STERILITY CHALLENGES AND INFECTION RISKS IN PPH DEVICES
7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE
7.1 OVERVIEW
7.2 UTERINE BALLOON TAMPONADE
7.2.1 BAKRI BALLOON
7.2.1.1 BAKRI POSTPARTUM BALLOON
7.2.1.2 BAKRI POSTPARTUM BALLOON WITH RAPID INSTILLATION COMPONENTS
7.2.2 FOLEY CATHETER
7.2.2.1 STANDARD FOLEY CATHETER
7.2.2.2 CONDOM-LOADED FOLEY CATHETER
7.3 UNIJECT PREFILLED INJECTION SYSTEM
7.3.1 OXYTOCIN-BASED INJECTION SYSTEM
7.3.2 CARBETOCIN-BASED INJECTION SYSTEM
7.4 NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1 STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1.1 MEDIUM
7.4.1.2 LARGE
7.4.2 MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.2.1 MEDIUM
7.4.2.2 LARGE
7.5 VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES
7.5.1 JADA SYSTEM
7.5.2 OTHERS
7.6 OTHERS
7.6.1 COMPRESSION DEVICES
7.6.1.1 B-LYNCH
7.6.1.2 HAYMAN
7.6.1.3 OTHER
7.6.2 UTERINE ARTERY LIGATION PRODUCTS
7.6.3 OTHERS
8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE
8.1 OVERVIEW
8.2 PRIMARY PPH
8.3 SECONDARY PPH
9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION
9.1 OVERVIEW
9.2 MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML)
9.3 MINOR POSTPARTUM HEMORRHAGE (500-1000 ML)
9.4 MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE)
9.5 SECONDARY POSTPARTUM HEMORRHAGE
10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 RETAIL SALES
10.3.1 OFFLINE SALES
10.3.2 ONLINE SALES
10.4 OTHERS
11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.1.1 TIER 2
11.2.1.2 TIER 3
11.2.1.3 TIER 1
11.2.2 PRIVATE HOSPITALS
11.2.2.1 TIER 2
11.2.2.2 TIER 3
11.2.2.3 TIER 1
11.3 MATERNITY CENTERS
11.4 SPECIALTY CLINICS
11.5 HOME CARE SETTINGS
11.6 OTHERS
12 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 TURKEY
12.1.8 NETHERLAND
12.1.9 POLAND
12.1.10 BELGIUM
12.1.11 SWITZERLAND
12.1.12 SWEDEN
12.1.13 DENMARK
12.1.14 NORWAY
12.1.15 FINLAND
12.1.16 REST OF EUROPE
13 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 SOLUTION PORTFOLIO
15.1.5 RECENT NEWS
15.2 ORGANON GROUP OF COMPANIES
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS/NEWS
15.3 LABORIE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 COOPERCOMPANIES
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 BELMONT MEDICAL TECHNOLOGIES
15.5.1 COMPANY SNAPSHOT
15.5.2 COMPANY SHARE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENT
15.6 ADVIN HEALTH CARE
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 ANGIPLAST PRIVATE LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 COAGULANT THERAPEUTICS
15.8.1 COMPANY SNAPSHOT
15.8.2 PIPELINE PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 KRISHCO MEDICAL PRODUCTS PVT. LTD
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 MATERNOVA INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 REVMEDX
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 3RD STONE DESIGN
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 STERIMED GROUP
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.14 UTAH MEDICAL PRODUCTS, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SINAPI BIOMEDICAL
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
Lista de Tabela
TABLE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE PRIMARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SECONDARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE MINOR POSTPARTUM HEMORRHAGE (500-1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SECONDARY POSTPARTUM HEMORRHAGE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE DIRECT TENDER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE MATERNITY CENTERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE SPECIALTY CLINICS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE HOME CARE SETTINGS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GERMANY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GERMANY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 GERMANY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 GERMANY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 GERMANY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GERMANY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 66 GERMANY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 67 GERMANY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 GERMANY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GERMANY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 71 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 GERMANY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 GERMANY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GERMANY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 77 GERMANY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 FRANCE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 FRANCE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 FRANCE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 FRANCE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 FRANCE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 FRANCE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 85 FRANCE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 86 FRANCE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 FRANCE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 FRANCE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 90 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 92 FRANCE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 FRANCE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 FRANCE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 FRANCE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 U.K. UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 U.K. BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 U.K. FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 U.K. UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 U.K. NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 U.K. STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 104 U.K. MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 105 U.K. VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 U.K. OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 U.K. COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 109 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 U.K. HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 U.K. PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 U.K. PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 115 U.K. RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 ITALY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 ITALY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 ITALY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 ITALY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 ITALY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 ITALY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 123 ITALY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 124 ITALY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 ITALY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 ITALY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 128 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 130 ITALY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 131 ITALY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 ITALY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 134 ITALY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 SPAIN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 SPAIN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 SPAIN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 SPAIN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 SPAIN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 SPAIN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 142 SPAIN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 143 SPAIN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 SPAIN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 SPAIN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 147 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 149 SPAIN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 SPAIN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 SPAIN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 153 SPAIN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 RUSSIA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 RUSSIA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 RUSSIA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 RUSSIA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 RUSSIA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 RUSSIA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 161 RUSSIA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 162 RUSSIA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 RUSSIA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 RUSSIA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 166 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 168 RUSSIA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 RUSSIA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 RUSSIA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 172 RUSSIA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 TURKEY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 TURKEY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 TURKEY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 TURKEY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 TURKEY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 TURKEY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 180 TURKEY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 181 TURKEY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 TURKEY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 TURKEY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 185 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 187 TURKEY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 TURKEY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 TURKEY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 191 TURKEY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 NETHERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 NETHERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 NETHERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 NETHERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 NETHERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 NETHERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 199 NETHERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 200 NETHERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 NETHERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 NETHERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 204 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 206 NETHERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 NETHERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 NETHERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 210 NETHERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 POLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 213 POLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 214 POLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 POLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 POLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 POLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 218 POLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 219 POLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 POLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 POLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 223 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 225 POLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 POLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 POLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 229 POLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 BELGIUM UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 BELGIUM BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 BELGIUM FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 BELGIUM UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 235 BELGIUM NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 BELGIUM STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 237 BELGIUM MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 238 BELGIUM VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 BELGIUM OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 BELGIUM COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 242 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 244 BELGIUM HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 BELGIUM PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 BELGIUM PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 247 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 248 BELGIUM RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 SWITZERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 SWITZERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 SWITZERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 SWITZERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 SWITZERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 SWITZERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 256 SWITZERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 257 SWITZERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 SWITZERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 SWITZERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 261 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 263 SWITZERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 SWITZERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 SWITZERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 267 SWITZERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 SWEDEN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 SWEDEN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 SWEDEN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 SWEDEN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 SWEDEN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 274 SWEDEN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 275 SWEDEN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 276 SWEDEN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 SWEDEN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 SWEDEN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 280 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 282 SWEDEN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 SWEDEN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 SWEDEN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 286 SWEDEN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 DENMARK UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 DENMARK BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 DENMARK FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 DENMARK UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 DENMARK NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 DENMARK STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 294 DENMARK MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 295 DENMARK VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 DENMARK OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 DENMARK COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 299 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 301 DENMARK HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 DENMARK PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 DENMARK PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 305 DENMARK RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NORWAY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NORWAY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 309 NORWAY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 NORWAY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NORWAY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 NORWAY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 313 NORWAY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 314 NORWAY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 NORWAY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 NORWAY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 318 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 320 NORWAY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NORWAY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 NORWAY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 324 NORWAY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 326 FINLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 FINLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 FINLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 FINLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 FINLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 FINLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 332 FINLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 333 FINLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 334 FINLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 FINLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 337 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 338 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 339 FINLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 340 FINLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 341 FINLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 343 FINLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 344 REST OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Lista de Figura
FIGURE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE IS EXPECTED TO DRIVE THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 UTERINE BALLOON TAMPONADE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 15 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE (2024)
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
FIGURE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2024
FIGURE 18 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2024
FIGURE 22 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 23 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, CAGR (2025-2032)
FIGURE 24 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2024
FIGURE 26 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, CAGR (2025-2032)
FIGURE 28 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, LIFELINE CURVE
FIGURE 29 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 32 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 33 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2024
FIGURE 34 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY SHARE 2024 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.