Europe Stroke Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
9.72 Billion
USD
16.55 Billion
2024
2032
USD
9.72 Billion
USD
16.55 Billion
2024
2032
| 2025 –2032 | |
| USD 9.72 Billion | |
| USD 16.55 Billion | |
|
|
|
|
Segmentação do mercado de AVC na Europa, tipo (AVC isquêmico, ataque isquêmico transitório (AIT) e AVC hemorrágico ), diagnóstico e tratamento (diagnóstico e tratamento), gênero (feminino e masculino), usuário final (hospitais e clínicas, clínicas especializadas, centros cirúrgicos ambulatoriais, atendimento domiciliar, laboratórios e outros), canal de distribuição (direto, varejo e online) – tendências do setor e previsão até 2032
Análise do mercado de AVC na Europa
O mercado europeu de AVC é um setor em rápido crescimento dentro do setor de saúde, focado em produtos e serviços voltados para a prevenção, diagnóstico, tratamento e reabilitação de pacientes com AVC. Os principais componentes desse mercado incluem produtos farmacêuticos (como trombolíticos, antiplaquetários e anticoagulantes), dispositivos médicos (como stents vasculares e dispositivos neuroprotetores) e equipamentos de reabilitação (incluindo instrumentos de fisioterapia e fonoaudiologia). O crescimento é impulsionado por fatores como a crescente conscientização sobre a prevenção do AVC, os avanços na tecnologia médica e o aumento da população geriátrica, que é mais suscetível a AVC. A crescente prevalência de doenças crônicas não transmissíveis, particularmente hipertensão e diabetes, contribui ainda mais para a demanda por cuidados eficazes para o AVC.
Tamanho do mercado de AVC
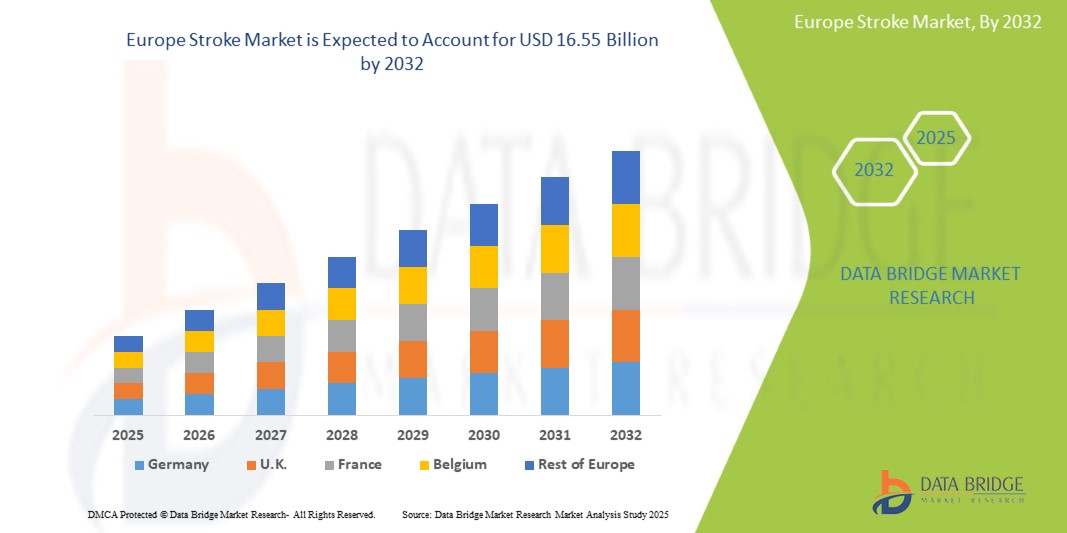
Espera-se que o mercado europeu de AVC atinja US$ 16,55 bilhões até 2032, ante US$ 9,72 bilhões em 2024, crescendo a um CAGR de 7,2% no período previsto de 2025 a 2032. Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de pipeline, análise de preços e estrutura regulatória.
Tendências do mercado de AVC
“Adoção crescente de tecnologias avançadas de neuroimagem e soluções de telemedicina para diagnóstico e tratamento de AVC”
Uma tendência notável no mercado europeu de AVC é a crescente adoção de tecnologias avançadas de neuroimagem e soluções de telemedicina para diagnóstico e tratamento de AVC. À medida que cresce a conscientização sobre a importância crucial da intervenção rápida no tratamento de AVC, observa-se uma mudança significativa em direção à utilização de técnicas sofisticadas de imagem, como ressonância magnética e tomografia computadorizada, que permitem um diagnóstico mais rápido e preciso dos tipos de AVC. Além disso, a telemedicina está se tornando essencial para fornecer consultas oportunas e monitoramento remoto, especialmente em áreas rurais ou carentes, permitindo que os profissionais de saúde avaliem os pacientes e iniciem os tratamentos prontamente. Essa tendência não apenas melhora os resultados dos pacientes, mas também impulsiona a inovação e o investimento no tratamento contínuo do AVC.
Escopo do Relatório e Segmentação do Mercado de AVC
|
Atributos |
Principais insights de mercado sobre AVC |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Alemanha, França, Reino Unido, Holanda, Suíça, Rússia, Itália, Espanha, Turquia, Áustria, Polônia, Noruega, Irlanda e Resto da Europa |
|
Principais participantes do mercado |
Bristol-Myers Squibb Company (EUA), Boehringer Ingelheim International GmbH (Alemanha), F. Hoffmann-La Roche Ltd (Suíça), DAIICHI SANKYO COMPANY, LIMITED (Japão), Sanofi (França), Johnson & Johnson Services, Inc. (EUA), Bayer AG (Alemanha), Sandoz AG (Suíça), Pfizer Inc. (EUA), Medtronic (Irlanda), Abbott (EUA), Viatris Inc. (EUA), AstraZeneca (Reino Unido), Penumbra, Inc. (EUA), GLENMARK PHARMACEUTICALS LTD (Índia), Fresenius SE & Co. KGaA (Alemanha), Teva Pharmaceuticals USA, Inc. (Israel), Lupin (Índia) e Amneal Pharmaceuticals LLC (EUA), entre outras. |
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de pipeline, análise de preços e estrutura regulatória. |
Definição de Mercado de AVC
O mercado europeu de AVC abrange os diversos produtos, serviços e tecnologias envolvidos na prevenção, diagnóstico, tratamento e reabilitação de pacientes com AVC em todo o mundo. Isso inclui uma gama de dispositivos médicos, produtos farmacêuticos, equipamentos de imagem e soluções terapêuticas voltadas para a complexidade do tratamento do AVC. O mercado é impulsionado pelo aumento da incidência de AVC devido ao envelhecimento da população, fatores relacionados ao estilo de vida e à maior conscientização sobre os sintomas e opções de tratamento do AVC. Além disso, os avanços nas tecnologias de saúde e na telemedicina estão moldando o cenário, facilitando um melhor gerenciamento do paciente e melhorando os resultados no tratamento do AVC em diversos grupos demográficos e contextos de saúde.
Dinâmica do Mercado de Acidentes Vasculares Cerebrais
Motoristas
- Aumento da incidência de AVC aumenta demanda por tratamentos
O aumento da incidência de AVC é um fator significativo no mercado de AVC, influenciando tanto a demanda por tratamento quanto a infraestrutura de saúde. O AVC, uma das principais causas de incapacidade e morte em todo o mundo, é cada vez mais comum devido a diversos fatores de risco, incluindo envelhecimento da população, sedentarismo, hipertensão arterial, diabetes, tabagismo e má alimentação. À medida que a expectativa de vida aumenta e a população envelhece, a prevalência de condições que contribuem para o AVC, como hipertensão e fibrilação atrial, também aumenta, resultando em mais pessoas sofrendo AVC e necessitando de atenção médica imediata e reabilitação a longo prazo.
Por exemplo,
Em maio de 2023, de acordo com o artigo publicado na eClinicalMedicine, o AVC era a segunda principal causa de morte e a terceira principal causa de incapacidade em todo o mundo. Nos últimos 30 anos, houve um aumento no número absoluto de AVC incidentes (70%) e prevalentes (85%), bem como de mortes (43%) por AVC.
- Número crescente de pacientes com hipertensão e doenças coronárias
Hipertensão, comumente conhecida como pressão alta, é uma condição médica caracterizada pela elevada pressão do sangue contra as paredes das artérias. Ela é tipicamente definida por uma leitura de pressão arterial de 130/80 mm Hg ou superior e pode ser categorizada como essencial (primária) ou secundária, dependendo da sua causa subjacente. A hipertensão prolongada pode levar a uma série de problemas de saúde graves, sendo um dos mais significativos a doença arterial coronariana. A doença arterial coronariana, também conhecida como doença arterial coronariana, resulta do acúmulo gradual de depósitos de gordura (aterosclerose) nas artérias coronárias, que fornecem oxigênio e nutrientes ao músculo cardíaco. À medida que essas artérias se estreitam ou ficam bloqueadas, o fluxo sanguíneo para o coração é reduzido, levando a dor no peito (angina) e, em casos graves, ataques cardíacos.
Por exemplo,
Em setembro de 2023, de acordo com um artigo publicado no Pan American Health Organization Journal, a hipertensão, frequentemente assintomática, contribui significativamente para as doenças cardiovasculares, que são a principal causa de morte. Fatores como envelhecimento, obesidade e escolhas inadequadas de estilo de vida estão impulsionando o aumento da prevalência, exigindo tratamentos eficazes.
Oportunidades
- Desenvolvimento de Terapêuticas Avançadas para Acidentes Vasculares Cerebrais
O desenvolvimento de terapias avançadas representa uma oportunidade significativa para o mercado de AVC, ao atender às substanciais necessidades não atendidas no tratamento do AVC. Os tratamentos atuais, focados principalmente na restauração do fluxo sanguíneo por meio de trombólise ou trombectomia, são eficazes apenas em um curto espaço de tempo e não tratam o dano neuronal subjacente. Terapias avançadas, como agentes neuroprotetores, terapias celulares e sistemas de administração direcionada de fármacos, prometem mitigar esse dano, promover o reparo neuronal e melhorar os resultados funcionais a longo prazo para pacientes com AVC. Isso levará à redução da incapacidade, à redução dos custos de saúde associados aos cuidados de longa duração e à melhoria da qualidade de vida dos sobreviventes, expandindo assim o potencial de mercado, atraindo investimentos e impulsionando a demanda por tratamentos mais eficazes.
Por exemplo,
Em abril de 2022, de acordo com um artigo publicado pela American Heart Association Journals, o tratamento do AVC isquêmico agudo continua a avançar. O tenecteplase foi avaliado como um trombolítico alternativo e as evidências sugerem que ele é pelo menos tão eficaz quanto o alteplase, podendo lisar coágulos de grandes vasos com mais eficácia. A terapia endovascular com trombectomia mecânica demonstrou ser benéfica até 24 horas após o início do AVC em pacientes cuidadosamente selecionados com oclusões proximais de grandes vasos.
- Expansão dos Serviços de Reabilitação de AVC
A expansão dos serviços de reabilitação para AVC representa uma oportunidade substancial para o mercado de AVC, atendendo à crescente necessidade de programas de recuperação e reabilitação mais eficazes. Atualmente, sobreviventes de AVC frequentemente enfrentam desafios significativos para recuperar as funções motoras e cognitivas perdidas, o que leva a internações hospitalares prolongadas, aumento dos custos médicos e redução da qualidade de vida. À medida que a população global envelhece e as taxas de incidência de AVC aumentam, há uma necessidade urgente de serviços de reabilitação aprimorados que atendam às necessidades individuais dos sobreviventes de AVC. Ao expandir os serviços de reabilitação para AVC, os provedores de saúde e os pagadores podem atender à demanda não atendida por cuidados abrangentes e personalizados, resultando em melhores resultados para os pacientes, redução dos custos com saúde e aumento da satisfação do paciente.
Por exemplo,
Em abril de 2023, de acordo com um artigo publicado pelo MDPI, países desenvolvidos se esforçam para oferecer reabilitação a pacientes com AVC. A reabilitação física pode reduzir ou prevenir complicações conhecidas em pacientes com AVC, além de melhorar sua qualidade de vida. Os terapeutas escolhem as intervenções com base em deficiências, limitações de atividade e objetivos de recuperação.
Restrições/Desafios
- Alto custo do diagnóstico
Doenças cardíacas e AVC são um dos principais fatores que contribuem para o aumento da taxa de mortalidade global ao longo dos anos. O AVC pode ser classificado como uma das doenças crônicas mais custosas. Mais de 868.000 americanos morrem de doenças cardíacas ou AVC todos os anos — o que representa um terço de todas as mortes. Com o aumento da incidência de AVC, o custo do diagnóstico e do tratamento tem aumentado ao longo dos anos, o que representa o principal fator limitante.
A maioria dos pacientes não só sofre com incapacidades permanentes que afetam seus meios de subsistência, como também tem um enorme impacto econômico na sociedade. O custo do diagnóstico também aumentou com o avanço tecnológico.
Por exemplo,
De acordo com a Agência de Pesquisa e Qualidade em Assistência Médica, a internação hospitalar média para acidente vascular cerebral isquêmico (que inclui diagnóstico e internação) é de 5,6 dias a US$ 9.100 por internação, e para acidente vascular cerebral hemorrágico, é de 8,4 dias a US$ 19.500 por internação.
- Aumento do recall de produtos
Uma ampla gama de dispositivos de diagnóstico para AVC é utilizada por profissionais para realizar diferentes procedimentos em pacientes de diferentes faixas etárias. Portanto, os efeitos colaterais e complicações associados ao uso desses dispositivos podem causar sérios danos aos pacientes.
Além disso, esses dispositivos e produtos de diagnóstico são muito caros e altamente arriscados, e uma possível falha pode causar consequências graves ao paciente. Portanto, são rigorosamente regulamentados e recolhidos para a segurança dos pacientes.
Por exemplo,
O produto da empresa Neusoft Medical Systems Co., Ltd., NeuViz 64 Multi-slice CT Scanner System, que é um sistema de tomografia computadorizada multislice usado como um sistema de raio-X de tomografia computadorizada de corpo inteiro com um tubo de raio-X em rotação contínua e um conjunto de detectores, foi recolhido pelo FDA devido a um erro de software no sistema.
Este relatório de mercado fornece detalhes sobre novos desenvolvimentos recentes, regulamentações comerciais, análise de importação e exportação, análise de produção, otimização da cadeia de valor, participação de mercado, impacto de participantes do mercado doméstico e local, análise de oportunidades em termos de bolsões de receita emergentes, mudanças nas regulamentações de mercado, análise estratégica de crescimento de mercado, tamanho de mercado, crescimento de categorias de mercado, nichos de aplicação e dominância, aprovações de produtos, lançamentos de produtos, expansões geográficas e inovações tecnológicas no mercado. Para obter mais informações sobre o mercado, entre em contato com a Data Bridge Market Research para um Briefing de Analista. Nossa equipe ajudará você a tomar uma decisão de mercado informada para alcançar o crescimento do mercado.
Escopo do mercado de AVC
O mercado é categorizado em cinco segmentos notáveis com base em tipo, diagnóstico e tratamento, gênero, usuário final e canal de distribuição. O crescimento entre esses segmentos ajudará você a analisar os segmentos de crescimento escasso nos setores e fornecerá aos usuários uma visão geral e insights valiosos do mercado para ajudá-los a tomar decisões estratégicas para identificar as principais aplicações de mercado.
Tipo
- Acidente vascular cerebral isquêmico
- Trombótica (Trombose Cerebral)
- Embólico (Embolia Cerebral)
- AVC hemorrágico
- Hemorragia Subaracnóidea
- Hemorragia Intracerebral
- Ataque Isquêmico Transitório (AIT)
Diagnóstico e Tratamento
- Tratamento
- Medicamento
- Por classe
- Medicamentos para pressão arterial
- Inibidores da enzima conversora de angiotensina (ECA)
- Ramipril
- Lisinopril
- Enalapril
- Perindopril
- Outro
- Diuréticos tiazídicos
- Indapamida
- Bendroflumetiazida
- Espironolactona
- Amilorida
- Outro
- Bloqueadores dos canais de cálcio
- Amlodipina
- Nifedipina
- Verapamil
- Nicardipina
- Felodipina
- Nimodipina
- Outro
- Betabloqueadores
- Atenolol
- Bisoprolol
- Labetolol
- Outros
- Bloqueadores Alfa
- Doxazosina
- Outros
- Outros
- Inibidores da enzima conversora de angiotensina (ECA)
- Medicamentos antiplaquetários
- Aspirina
- Clopidogrel
- Dipiridamol
- Ticlopidina
- Outros
- Anticoagulantes
- Varfarina
- Apixabana
- Dabigatrana
- Heparina
- Rivaroxabana
- Outro
- Ativador de Plasminogênio Tecidual (TPA)
- Alteplase
- Tenecteplase
- Reteplase
- Anistreplase
- Outro
- Estatinas
- Atorvastatina
- Sinvastatina
- Lovastatina
- Rosuvastatina
- Fluvastatina
- Pravastatina
- Pitavastatina
- Outros
- Vitamina K
- Medicação de suporte
- Suplementos Nutricionais
- Antipiréticos
- Outros
- Medicamentos para pressão arterial
- Por tipo de medicamento
- De marca
- Ativar
- Edobaxan
- Coumadin
- Heparina Leo
- Duoplavina
- Aggrenox
- Retavase
- Jantoven
- Cathflo
- Outro
- De marca
- Genérico
- Por via de administração
- Oral
- Comprimido
- Cápsulas
- Outros
- Parenteral
- Intravenoso
- Subcutâneo
- Outros
- Oral
- Por Modo de Compra
- Prescrição
- Sem receita médica (OTC)
- Por tipo de terapia
- Terapia Combinada
- Monoterapia
- Por via de administração
- Cirurgia
- Bobinas Embólicas
- Cateteres de Aspiração
- Recuperador de Stent
- Clipagem Cirúrgica
- Outros
- Outros Terapia
- Fisioterapia
- Terapia ocupacional
- Terapia da Fala
- Outros
- Por classe
- Diagnóstico
- Teste de imagem
- Tomografia Computadorizada (TC)
- Imagem por ressonância magnética (RM)
- Ultrassom de carótida
- Angiograma Cerebral
- Exame de sangue
- Ecocardiograma
- Punção Lombar
- Outros
- Teste de imagem
- Medicamento
Gênero
- Fêmea
- Macho
Usuário final
- Hospitais e Clínicas
- Clínicas especializadas
- Centro Cirúrgico Ambulatorial
- Assistência domiciliar
- Laboratórios
- Outros
Canal de Distribuição
- Direto
- Varejo
- On-line
Análise regional do mercado de AVC
O mercado é analisado e insights sobre o tamanho do mercado e tendências são fornecidos por tipo, diagnóstico e tratamento, gênero, usuário final e canal de distribuição, conforme referenciado acima.
Os países abrangidos pelo mercado são Alemanha, França, Reino Unido, Holanda, Suíça, Rússia, Itália, Espanha, Turquia, Áustria, Polônia, Noruega, Irlanda e Resto da Europa.
Espera-se que a Alemanha domine e seja o país com crescimento mais rápido no mercado de AVC na Europa devido aos seus altos gastos com saúde, infraestrutura médica avançada e programas abrangentes de tratamento de AVC.
A seção sobre países do relatório também apresenta fatores individuais que impactam o mercado e mudanças na regulamentação do mercado doméstico, que impactam as tendências atuais e futuras do mercado. Pontos de dados como análise da cadeia de valor a montante e a jusante, tendências técnicas, análise das cinco forças de Porter e estudos de caso são alguns dos indicadores utilizados para prever o cenário de mercado para cada país. Além disso, a presença e a disponibilidade de marcas europeias e seus desafios enfrentados devido à concorrência forte ou escassa de marcas locais e nacionais, o impacto de tarifas domésticas e rotas comerciais são considerados na análise de previsão dos dados do país.
Participação no mercado de AVC
O cenário competitivo do mercado fornece detalhes por concorrente. Os detalhes incluem visão geral da empresa, finanças da empresa, receita gerada, potencial de mercado, investimento em pesquisa e desenvolvimento, novas iniciativas de mercado, presença na Europa, locais e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento de produto, abrangência e amplitude do produto e domínio da aplicação. Os dados fornecidos acima referem-se apenas ao foco das empresas em relação ao mercado.
Os líderes de mercado de AVC que operam no mercado são:
- Bristol-Myers Squibb Company (EUA)
- Boehringer Ingelheim International GmbH (Alemanha)
- F. Hoffmann-La Roche Ltd (Suíça)
- DAIICHI SANKYO COMPANY, LIMITED (Japão)
- Sanofi (França)
- Johnson & Johnson Services, Inc. (EUA)
- Bayer AG (Alemanha)
- Sandoz AG (Suíça)
- Pfizer Inc. (EUA)
- Medtronic (Irlanda)
- Abbott (EUA)
- Viatris Inc. (EUA)
- AstraZeneca (Reino Unido)
- Penumbra, Inc. (EUA)
- GLENMARK PHARMACEUTICALS LTD (Índia)
- Fresenius SE & Co. KGaA (Alemanha)
- Teva Pharmaceuticals USA, Inc. (Israel)
- Lupin (Índia)
- Amneal Pharmaceuticals LLC (EUA)
Últimos desenvolvimentos no mercado de AVC
- Em julho de 2023, a Roche anunciou uma nova parceria com a Alnylam para desenvolver e comercializar o zilebesir, uma terapia de RNAi experimental, atualmente em Fase 2, para o tratamento da hipertensão arterial. Esta colaboração combina a experiência comprovada da Alnylam em terapia de RNAi com o alcance comercial da Roche na Europa, seu compromisso com a inovação e seu desejo de mudar o cenário para pacientes com doenças cardiovasculares graves.
- Em setembro de 2020, a Daiichi Sankyo Company Limited anunciou a submissão de um pedido suplementar no Japão para a aprovação estendida do anticoagulante edoxabana (hidrato de benzoato de edoxabana) para pacientes idosos com regurgitação não valvar e sangramento grave. Risco. Este pedido baseia-se nos resultados de um ensaio clínico japonês de Fase 3 (ensaio ELDERCARE-AF) em 984 pacientes com fibrilação atrial não valvar, com pelo menos 80 anos de idade, alto risco de sangramento e sem indicação para outras terapias anticoagulantes disponíveis. A Daiichi Sankyo planeja contribuir para o tratamento de pacientes idosos com fibrilação atrial não valvar, oferecendo uma nova opção de tratamento.
- Em julho de 2022, a Sandoz, fabricante líder mundial de genéricos e biossimilares, anunciou um investimento de aproximadamente US$ 90 milhões em sua unidade em Liubliana, Eslovênia, para estabelecer seu Centro de Desenvolvimento de Biofármacos Sandoz até 2026. Com esse investimento, a unidade de Liubliana se tornará uma das mais importantes unidades de desenvolvimento de biossimilares da Sandoz. O novo escritório resultará na criação de aproximadamente 200 novos empregos em tempo integral e fortalecerá ainda mais as capacidades da empresa no desenvolvimento de biossimilares e produtos farmacêuticos.
- Em janeiro de 2023, a Penumbra, Inc., empresa europeia de saúde focada em terapias inovadoras, anunciou a aprovação e o lançamento do Lightning Flash™ pela Food and Drug Administration (FDA) dos EUA, o sistema de trombectomia mecânica mais avançado e potente do mercado. O Lightning Flash apresenta a nova tecnologia de Aspiração Inteligente Lightning da Penumbra, que agora conta com dois algoritmos de detecção de coágulos. Combinado com a inovadora tecnologia de cateter, o Lightning Flash foi projetado para remover rapidamente grandes coágulos sanguíneos do corpo, incluindo embolia venosa e embolia pulmonar (EP). Este lançamento ajudará a empresa a expandir seu portfólio de produtos, pois os resultados avançados dessa nova tecnologia são excepcionalmente rastreáveis e sua capacidade única de diferenciar sangue circulante de coágulos.
- Em agosto de 2023, a Lupin anunciou o lançamento do Jeet, um programa de apoio ao paciente dedicado à saúde cardíaca. O lançamento da iniciativa coincide com o 77º Dia da Independência da Índia, que simboliza a libertação do estresse relacionado a doenças e a jornada para uma vida mais feliz e saudável. O Jeet se torna um parceiro confiável em cuidados cardiovasculares, oferecendo diversos benefícios, como redução de custos, assistência médica, lembretes de medicamentos e suporte ao estilo de vida. O Jeet oferece uma abordagem holística para melhorar a experiência do médico e do paciente, aumentando a conscientização sobre doenças cardiovasculares e suas comorbidades. O aplicativo inclui recursos projetados para incentivar um estilo de vida mais saudável e apoiar um coração saudável.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 LIMITATIONS
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 EUROPE STROKE MARKET, REGULATORY FRAMEWORK
5.1 REGULATION IN U.S.
5.2 REGULATION IN EUROPE
5.3 REGULATION IN CHINA
5.4 REGULATION IN JAPAN
5.5 REGULATION IN SOUTH AFRICA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS
6.1.2 INCREASING NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
6.1.3 INCREASING DIABETIC AND OBESE POPULATIONS ELEVATE STROKE RISKS
6.1.4 ADVANCEMENTS IN MEDICAL TECHNOLOGY IMPROVE STROKE CARE OUTCO.MES
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSIS
6.2.2 INCREASE IN PRODUCT RECALL
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF ADVANCED THERAPEUTICS FOR STROKES
6.3.2 EXPANSION IN STROKE REHABILITATION SERVICES
6.3.3 INNOVATIVE TREATMENTS IN PIPELINE FOR STROKE TREATMENT
6.4 CHALLENGES
6.4.1 FALSE DIAGNOSIS IN STROKES
6.4.2 COMPLICATIONS ASSOCIATED WITH MANAGING STROKE
7 EUROPE STROKE MARKET, BY TYPE
7.1 OVERVIEW
7.2 ISCHEMIC STROKE
7.2.1 THROMBOTIC (CEREBRAL THROMBOSIS)
7.2.2 EMBOLIC (CEREBRAL EMBOLISM)
7.3 HEMORRHAGIC STROKE
7.3.1 SUBARACHNOID HEMORRHAGE
7.3.2 INTRACEREBRAL HEMORRHAGE
7.4 TRANSIENT ISCHEMIC ATTACT (TIA)
8 EUROPE STROKE MARKET, BY GENDER
8.1 OVERVIEW
8.2 FEMALE
8.3 MALE
9 EUROPE STROKE MARKET, BY DIAGNOSIS AND TREATMENT
9.1 OVERVIEW
9.2 TREATMENT
9.2.1 BY TREATMENT TYPE
9.2.1.1 MEDICATION
9.2.1.1.1 MEDICATION, BY CLASS
9.2.1.1.1.1 BLOOD PRESSURE MEDICINES
9.2.1.1.1.1.1 ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS
9.2.1.1.1.1.2 RAMIPRIL
9.2.1.1.1.1.3 LISINOPRIL
9.2.1.1.1.1.4 ENALAPRIL
9.2.1.1.1.1.5 PERINDOPRIL
9.2.1.1.1.1.6 OTHER
9.2.1.1.1.2 THIAZIDE DIURETICS
9.2.1.1.1.2.1 INDAPAMIDE
9.2.1.1.1.2.2 BENDROFLUMETHIAZIDE
9.2.1.1.1.2.3 SPIRONOLACTONE
9.2.1.1.1.2.4 AMILORIDE
9.2.1.1.1.2.5 OTHER
9.2.1.1.1.3 CALCIUM CHANNEL BLOCKERS
9.2.1.1.1.3.1 AMLODIPINE
9.2.1.1.1.3.2 NIFEDIPINE
9.2.1.1.1.3.3 VERAPAMIL
9.2.1.1.1.3.4 NICARDIPINE
9.2.1.1.1.3.5 FELODIPINE
9.2.1.1.1.3.6 NIMODIPINE
9.2.1.1.1.3.7 OTHERS
9.2.1.1.1.4 BETA BLOCKERS
9.2.1.1.1.4.1 ATENOLOL
9.2.1.1.1.4.2 BISOPROLOL
9.2.1.1.1.4.3 LABETOLOL
9.2.1.1.1.4.4 OTHERS
9.2.1.1.1.5 ALPHA-BLOCKERS
9.2.1.1.1.5.1 DOXAZOSIN
9.2.1.1.1.5.2 OTHERS
9.2.1.1.1.6 OTHERS
9.2.1.1.1.7 ANTIPLATELET DRUGS
9.2.1.1.1.7.1 ASPIRIN
9.2.1.1.1.7.2 CLOPIDOGREL
9.2.1.1.1.7.3 DIPYRIDAMOLE
9.2.1.1.1.7.4 TICLOPIDINE
9.2.1.1.1.7.5 OTHERS
9.2.1.1.1.8 ANTICOAGULANTS
9.2.1.1.1.8.1 WARFARIN
9.2.1.1.1.8.2 APIXABAN
9.2.1.1.1.8.3 DABIGATRAN
9.2.1.1.1.8.4 HEPARIN
9.2.1.1.1.8.5 RIVAROXABAN
9.2.1.1.1.8.6 OTHERS
9.2.1.1.1.9 TISSUE PLASMINOGEN ACTIVATOR (TPA)
9.2.1.1.1.9.1 ALTEPLASE
9.2.1.1.1.9.2 TENECTEPLASE
9.2.1.1.1.9.3 RETEPLASE
9.2.1.1.1.9.4 ANISTREPLASE
9.2.1.1.1.9.5 OTHERS
9.2.1.1.1.10 STATINS
9.2.1.1.1.10.1.1 ATORVASTATIN
9.2.1.1.1.10.1.2 SIMVASTATIN
9.2.1.1.1.10.1.3 LOVASTATIN
9.2.1.1.1.10.1.4 ROSUVASTATIN
9.2.1.1.1.10.1.5 FLUVASTATIN
9.2.1.1.1.10.1.6 PRAVASTATIN
9.2.1.1.1.10.1.7 PITAVASTATIN
9.2.1.1.1.10.1.8 OTHERS
9.2.1.1.1.11 VITAMIN K
9.2.1.1.1.12 SUPPORTIVE MEDICATION
9.2.1.1.1.12.1 NUTRITIONAL SUPPLEMENTS
9.2.1.1.1.12.2 ANTIPYRETICS
9.2.1.1.1.12.3 OTHERS
9.2.1.1.2 MEDICATION, BY DRUG TYPE
9.2.1.1.2.1 BRANDED
9.2.1.1.2.1.1 ACTIVASE
9.2.1.1.2.1.2 EDOBAXAN
9.2.1.1.2.1.3 COUMADIN
9.2.1.1.2.1.4 HEPARIN LEO
9.2.1.1.2.1.5 DUOPLAVIN
9.2.1.1.2.1.6 AGGRENOX
9.2.1.1.2.1.7 RETAVASE
9.2.1.1.2.1.8 JANTOVEN
9.2.1.1.2.1.9 CATHFLO
9.2.1.1.2.1.10 OTHER
9.2.1.1.2.2 GENERIC
9.2.1.1.3 MEDICATION, BY ROUTE OF ADMINISTRATION
9.2.1.1.3.1 ORAL
9.2.1.1.3.1.1 TABLET
9.2.1.1.3.1.2 CAPSULES
9.2.1.1.3.1.3 OTHERS
9.2.1.1.3.2 PARENTERAL
9.2.1.1.3.2.1 INTRAVENOUS
9.2.1.1.3.2.2 SUBCUTANEOUS
9.2.1.1.3.3 OTHERS
9.2.1.1.4 MEDICATION, BY MODE OF PURCHASE
9.2.1.1.4.1 PRESCRIPTION
9.2.1.1.4.2 OVER THE COUNTER (OTC)
9.2.1.1.5 MEDICATION, BY THERAPY TYPE
9.2.1.1.5.1 COMBINATION THERAPY
9.2.1.1.5.2 MONOTHERAPY
9.2.1.2 SURGERY
9.2.1.2.1 EMBOLIC COILS
9.2.1.2.2 ASPIRATION CATHETERS
9.2.1.2.3 STENT RETRIEVER
9.2.1.2.4 SURGICAL CLIPPING
9.2.1.2.5 OTHERS
9.2.1.3 OTHERS THERAPY
9.2.1.3.1 PHYSICAL THERAPY
9.2.1.3.2 OCCUPATIONAL THERAPY
9.2.1.3.3 SPEECH THERAPY
9.2.1.3.4 OTHERS
9.3 DIAGNOSIS
9.3.1 IMAGING TEST
9.3.1.1 COMPUTERIZED TOMOGRAPHY (CT) SCAN
9.3.1.2 MAGNETIC RESONANCE IMAGING (MRI)
9.3.1.3 CAROTID ULTRASOUND
9.3.1.4 CEREBRAL ANGIOGRAM
9.3.2 BLOOD TEST
9.3.3 ECHOCARDIOGRAM
9.3.4 LUMBAR PUNCTURE
9.3.5 OTHERS
10 EUROPE STROKE MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT
10.3 RETAIL
10.4 ONLINE
11 EUROPE STROKE MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS & CLINICS
11.3 SPECIALTY CLINICS
11.4 AMBULATORY SURGICAL CENTER
11.5 HOMECARE
11.6 LABORATORIES
11.7 OTHERS
12 EUROPE STROKE MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 U.K.
12.1.3 FRANCE
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 NETHERLANDS
12.1.8 SWITZERLAND
12.1.9 TURKEY
12.1.10 AUSTRIA
12.1.11 POLAND
12.1.12 NORWAY
12.1.13 IRELAND
12.1.14 REST OF EUROPE
13 EUROPE STROKE MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BRISTOL-MYERS SQUIBB COMPANY
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 DAIICHI SANKYO COMPANY, LIMITED
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 SANOFI
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AMNEAL PHARMACEUTICALS LLC
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 ASTRAZENECA
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 BAYER AG
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 FRESENIUS SE & CO. KGAA
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 GLENMARK PHARMACEUTICALS LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 JOHNSON & JOHNSON SERVICES, INC.
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 LUPIN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENT
15.14 MEDTRONIC
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 PENUMBRA, INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 REVENUE ANALYSIS
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENT
15.16 PFIZER INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 SANDOZ AG
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 TEVA PHARMACEUTICALS USA, INC.
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 VIATRIS INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tabela
TABLE 1 EUROPE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE ISCHEMIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE HEMORRHAGIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE TRANSIENT ISCHEMIC ATTACT (TIA) IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE FEMALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE MALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE TREATMENT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)..
TABLE 12 EUROPE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)..
TABLE 14 EUROPE BLOOD PRESSURE MEDICINES IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE DIAGNOSIS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)…
TABLE 35 EUROPE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)…
TABLE 37 EUROPE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE DIRECT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE RETAIL IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE ONLINE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE HOSPITALS & CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE SPECIALTY CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE AMBULATORY SURGICAL CENTER IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE HOMECARE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)..
TABLE 46 EUROPE LABORATORIES IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE OTHERS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)..
TABLE 55 EUROPE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 59 EUROPE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 60 EUROPE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 61 EUROPE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 62 EUROPE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 63 EUROPE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 64 EUROPE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 65 EUROPE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 66 EUROPE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 EUROPE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 EUROPE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 69 EUROPE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 EUROPE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 71 EUROPE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 72 EUROPE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 73 EUROPE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 EUROPE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 EUROPE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 EUROPE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 77 EUROPE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 78 EUROPE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 79 EUROPE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 80 GERMANY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 GERMANY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 GERMANY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 GERMANY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 84 GERMANY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 GERMANY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 86 GERMANY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 87 GERMANY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 88 GERMANY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 89 GERMANY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 90 GERMANY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 91 GERMANY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 92 GERMANY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 93 GERMANY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 94 GERMANY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 95 GERMANY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 96 GERMANY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 97 GERMANY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 GERMANY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 GERMANY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 100 GERMANY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 GERMANY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 102 GERMANY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 103 GERMANY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 104 GERMANY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 GERMANY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 GERMANY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 GERMANY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 GERMANY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 109 GERMANY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 110 GERMANY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 111 U.K. STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 U.K. ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 113 U.K. HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 U.K. STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 115 U.K. TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 U.K. MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 117 U.K. BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 118 U.K. ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 119 U.K. THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 120 U.K. CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 121 U.K. BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)…
TABLE 122 U.K. ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 123 U.K. ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 124 U.K. ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 125 U.K. TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 126 U.K. STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 127 U.K. SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 128 U.K. MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)..
TABLE 129 U.K. BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 130 U.K. MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 131 U.K. ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 U.K. PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 U.K. MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 134 U.K. MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 U.K. SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 U.K. OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 U.K. DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 U.K. IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 U.K. STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 140 U.K. STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 141 U.K. STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 142 FRANCE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 143 FRANCE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 FRANCE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 FRANCE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 146 FRANCE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 147 FRANCE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)…
TABLE 148 FRANCE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 149 FRANCE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 150 FRANCE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 151 FRANCE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 152 FRANCE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 153 FRANCE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 154 FRANCE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 155 FRANCE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 156 FRANCE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 157 FRANCE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 158 FRANCE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 159 FRANCE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 FRANCE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 FRANCE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 162 FRANCE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 FRANCE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 FRANCE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 165 FRANCE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 FRANCE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 FRANCE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 168 FRANCE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 FRANCE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)…
TABLE 170 FRANCE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 171 FRANCE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 172 FRANCE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 173 ITALY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 ITALY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 ITALY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 ITALY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 177 ITALY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 ITALY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 179 ITALY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 180 ITALY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 181 ITALY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 182 ITALY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 183 ITALY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)..
TABLE 184 ITALY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 185 ITALY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 186 ITALY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 187 ITALY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 188 ITALY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 189 ITALY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 190 ITALY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 191 ITALY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 ITALY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 193 ITALY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 ITALY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 ITALY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 196 ITALY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 ITALY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 ITALY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 ITALY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 200 ITALY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 ITALY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 202 ITALY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 203 ITALY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 204 SPAIN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 SPAIN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 SPAIN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 SPAIN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 208 SPAIN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 SPAIN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 210 SPAIN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 211 SPAIN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 212 SPAIN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 213 SPAIN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 214 SPAIN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 215 SPAIN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 216 SPAIN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 217 SPAIN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 218 SPAIN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 219 SPAIN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 220 SPAIN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 221 SPAIN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 SPAIN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)..
TABLE 223 SPAIN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 224 SPAIN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 SPAIN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 SPAIN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 227 SPAIN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 SPAIN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 SPAIN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 230 SPAIN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 SPAIN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 SPAIN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 233 SPAIN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 234 SPAIN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)..
TABLE 235 RUSSIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 RUSSIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 237 RUSSIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 238 RUSSIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 239 RUSSIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 RUSSIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 241 RUSSIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 242 RUSSIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 243 RUSSIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 244 RUSSIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 245 RUSSIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 246 RUSSIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 247 RUSSIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 248 RUSSIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 249 RUSSIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 250 RUSSIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 251 RUSSIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 252 RUSSIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 RUSSIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 RUSSIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 255 RUSSIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 RUSSIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 RUSSIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 258 RUSSIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 RUSSIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 RUSSIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 261 RUSSIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 RUSSIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 263 RUSSIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 264 RUSSIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 265 RUSSIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 266 NETHERLANDS STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 267 NETHERLANDS ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 NETHERLANDS HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 NETHERLANDS STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 270 NETHERLANDS TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 NETHERLANDS MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 272 NETHERLANDS BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 273 NETHERLANDS ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 274 NETHERLANDS THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 275 NETHERLANDS CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 276 NETHERLANDS BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 277 NETHERLANDS ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 278 NETHERLANDS ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 279 NETHERLANDS ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 280 NETHERLANDS TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 281 NETHERLANDS STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 282 NETHERLANDS SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 283 NETHERLANDS MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 NETHERLANDS BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 NETHERLANDS MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 286 NETHERLANDS ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 NETHERLANDS PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 NETHERLANDS MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 289 NETHERLANDS MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 NETHERLANDS SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 NETHERLANDS OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 NETHERLANDS DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 NETHERLANDS IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 NETHERLANDS STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 295 NETHERLANDS STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 296 NETHERLANDS STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 297 SWITZERLAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 SWITZERLAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 299 SWITZERLAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 SWITZERLAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 301 SWITZERLAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 SWITZERLAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 303 SWITZERLAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 304 SWITZERLAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 305 SWITZERLAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 306 SWITZERLAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 307 SWITZERLAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 308 SWITZERLAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 309 SWITZERLAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 310 SWITZERLAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 311 SWITZERLAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 312 SWITZERLAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 313 SWITZERLAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 314 SWITZERLAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 SWITZERLAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 SWITZERLAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 317 SWITZERLAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 318 SWITZERLAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 SWITZERLAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 320 SWITZERLAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 SWITZERLAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 SWITZERLAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 SWITZERLAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 SWITZERLAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 SWITZERLAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 326 SWITZERLAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 327 SWITZERLAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 328 TURKEY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 TURKEY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 TURKEY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 TURKEY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 332 TURKEY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 TURKEY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 334 TURKEY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 335 TURKEY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 336 TURKEY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 337 TURKEY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 338 TURKEY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 339 TURKEY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 340 TURKEY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 341 TURKEY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 342 TURKEY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 343 TURKEY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 344 TURKEY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 345 TURKEY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 346 TURKEY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 347 TURKEY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 348 TURKEY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 349 TURKEY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 350 TURKEY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 351 TURKEY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 352 TURKEY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 353 TURKEY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 354 TURKEY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 355 TURKEY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)…
TABLE 356 TURKEY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 357 TURKEY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 358 TURKEY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 359 AUSTRIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 360 AUSTRIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 361 AUSTRIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 362 AUSTRIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 363 AUSTRIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 364 AUSTRIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)…
TABLE 365 AUSTRIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 366 AUSTRIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 367 AUSTRIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 368 AUSTRIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 369 AUSTRIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 370 AUSTRIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 371 AUSTRIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 372 AUSTRIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 373 AUSTRIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 374 AUSTRIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 375 AUSTRIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 376 AUSTRIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 377 AUSTRIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 378 AUSTRIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 379 AUSTRIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 380 AUSTRIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 381 AUSTRIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 382 AUSTRIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 383 AUSTRIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 384 AUSTRIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 AUSTRIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 386 AUSTRIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 387 AUSTRIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 388 AUSTRIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 389 AUSTRIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 390 POLAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 391 POLAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 392 POLAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 393 POLAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 394 POLAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 POLAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)..
TABLE 396 POLAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 397 POLAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 398 POLAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 399 POLAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 400 POLAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 401 POLAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 402 POLAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 403 POLAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 404 POLAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 405 POLAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 406 POLAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 407 POLAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 408 POLAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 409 POLAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 410 POLAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 411 POLAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 412 POLAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 413 POLAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 414 POLAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 415 POLAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 POLAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 417 POLAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 418 POLAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 419 POLAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 420 POLAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 421 NORWAY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 422 NORWAY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 423 NORWAY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 NORWAY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 425 NORWAY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 426 NORWAY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND).
TABLE 427 NORWAY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 428 NORWAY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 429 NORWAY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 430 NORWAY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 431 NORWAY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 432 NORWAY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 433 NORWAY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 434 NORWAY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 435 NORWAY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 436 NORWAY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 437 NORWAY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 438 NORWAY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 439 NORWAY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 440 NORWAY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 441 NORWAY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 442 NORWAY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 443 NORWAY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 444 NORWAY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 445 NORWAY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 446 NORWAY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 447 NORWAY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 448 NORWAY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)..
TABLE 449 NORWAY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 450 NORWAY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 451 NORWAY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 452 IRELAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 453 IRELAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 454 IRELAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 455 IRELAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 456 IRELAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 457 IRELAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)..
TABLE 458 IRELAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 459 IRELAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 460 IRELAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 461 IRELAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 462 IRELAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 463 IRELAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 464 IRELAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 465 IRELAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 466 IRELAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 467 IRELAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 468 IRELAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 469 IRELAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 470 IRELAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 471 IRELAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 472 IRELAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 473 IRELAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 474 IRELAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 475 IRELAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 476 IRELAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 477 IRELAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 478 IRELAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 479 IRELAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND).
TABLE 480 IRELAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 481 IRELAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 482 IRELAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 483 REST OF EUROPE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Lista de Figura
FIGURE 1 EUROPE STROKE MARKET: SEGMENTATION
FIGURE 2 EUROPE STROKE MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE STROKE MARKET: DROC ANALYSIS
FIGURE 4 EUROPE STROKE MARKET : EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE STROKE MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE STROKE MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE STROKE MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE STROKE MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE STROKE MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE STROKE MARKET: SEGMENTATION
FIGURE 11 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS IS EXPECTED TO DRIVE THE EUROPE STROKE MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 12 ISCHEMIC STROKE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE STROKE MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 13 EUROPE STROKE MARKET EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE STROKE MARKET
FIGURE 16 EUROPE STROKE MARKET: BY TYPE, 2024
FIGURE 17 EUROPE STROKE MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 18 EUROPE STROKE MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 19 EUROPE STROKE MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 EUROPE STROKE MARKET: BY GENDER, 2024
FIGURE 21 EUROPE STROKE MARKET: BY GENDER, 2025 TO 2032 (USD THOUSAND)
FIGURE 22 EUROPE STROKE MARKET: BY GENDER, CAGR (2025- 2032)
FIGURE 23 EUROPE STROKE MARKET: BY GENDER, LIFELINE CURVE
FIGURE 24 EUROPE STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2024
FIGURE 25 EUROPE STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2025-2032 (USD THOUSAND)
FIGURE 26 EUROPE STROKE MARKET: BY DIAGNOSIS AND TREATMENT, CAGR (2025-2032)
FIGURE 27 EUROPE STROKE MARKET: BY DIAGNOSIS AND TREATMENT, LIFELINE CURVE
FIGURE 28 EUROPE STROKE MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 29 EUROPE STROKE MARKET: BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
FIGURE 30 EUROPE STROKE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 31 EUROPE STROKE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 32 EUROPE STROKE MARKET: BY END USER, 2024
FIGURE 33 EUROPE STROKE MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 34 EUROPE STROKE MARKET: BY END USER, CAGR (2025-2032)
FIGURE 35 EUROPE STROKE MARKET: BY END USER, LIFELINE CURVE
FIGURE 36 EUROPE STROKE MARKET: SNAPSHOT (2024)
FIGURE 37 EUROPE STROKE MARKET: COMPANY SHARE 2024 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.