Europe Traumatic Brain Injury Treatment Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
1.01 Billion
USD
1.60 Billion
2024
2032
USD
1.01 Billion
USD
1.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.01 Billion | |
| USD 1.60 Billion | |
|
|
|
Europe Traumatic Brain Injury Treatment Market Segmentation, By Treatment (Surgery, Immediate Emergency Care, and Medications), Route of Administration (Parenteral, Oral, and Others), Patient Age (Children, Teenager, and Elder), Gender (Male and Female), Cause of Injury (Falls, Motor Vehicle Traffic, Sports, and Others), End User (Hospitals, Neurology Clinics, Independent Pharmacies, and Others) - Industry Trends and Forecast to 2032
Traumatic Brain Injury Treatment Market Size
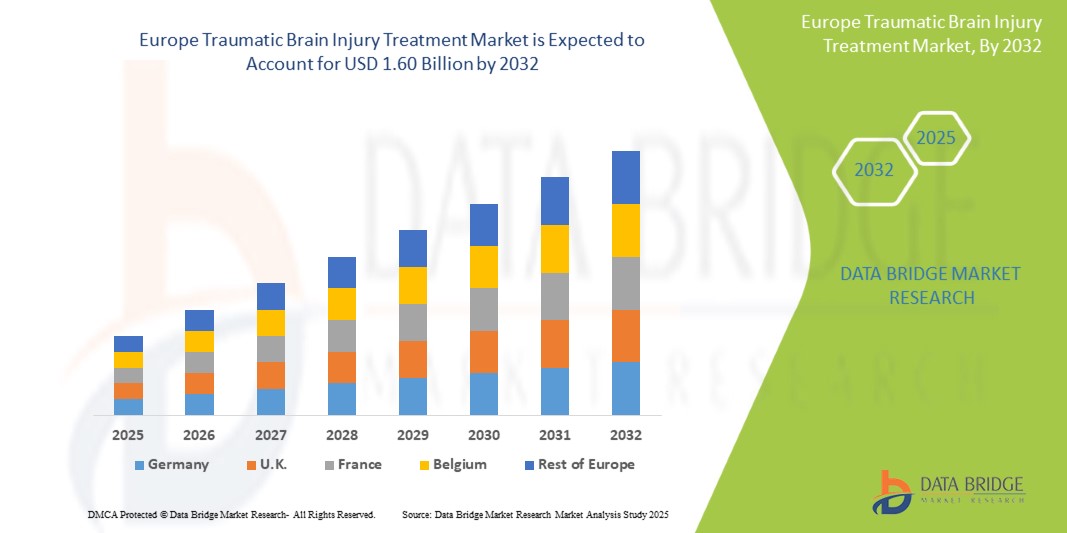
- The Europe traumatic brain injury treatment market was valued at USD 1.01 billion in 2024 and is expected to reach USD 1.60 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 6.0%, primarily driven by the increasing incidence of traumatic brain injury (TBI)
- This growth is driven by factors such as the increasing incidence of traumatic brain injury (TBI), growing adoption of minimally invasive procedures in TBI treatment, drives the demand for traumatic brain injury treatment
Traumatic Brain Injury Treatment Market Analysis
- The traumatic brain injury (TBI) treatment market is projected to expand significantly due to increasing awareness of TBI, advancements in diagnostic technologies, and the rising incidence of accidents and sports-related injuries, driving demand for effective treatment options and rehabilitation therapies
- The market is witnessing a surge in innovative treatments, including neuroprotective agents, stem cell therapies, and advanced rehabilitation technologies, which are enhancing recovery outcomes and expanding the therapeutic landscape for TBI patients
- Germany is projected to lead the Europe traumatic brain injury (TBI) treatment market, attributed to its advanced healthcare infrastructure and significant investments in medical research and development
Report Scope and Traumatic Brain Injury Treatment Market Segmentation
|
Attributes |
Traumatic Brain Injury Treatment Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Traumatic Brain Injury Treatment Market Trends
“Growing Adoption of Telemedicine in TBI Treatment”
- Telemedicine allows patients to receive remote consultations and rehabilitation services, improving access to specialized care for individuals in rural or underserved areas, thus facilitating timely intervention for TBI patients
- Utilizing telehealth reduces the costs associated with in-person visits, including travel expenses and lost productivity, making it a financially viable option for both patients and healthcare providers
- In March 2021, NCBI stated that Telehealth visits for patients with acquired brain injuries and their caregivers can ease the burden of transportation, improve compliance, and increase overall satisfaction. Management strategies are largely unaffected in the telehealth setting, and telerehab options have been found to be equal or superior to in-person therapy to treat many associated deficits
- Telemedicine platforms enable continuous monitoring and follow-up care, allowing healthcare professionals to track patient progress remotely, provide real-time feedback, and adjust treatment plans as necessary, ultimately enhancing patient outcomes in TBI management
Traumatic Brain Injury Treatment Market Dynamics
Driver
“Increasing Incidence of Traumatic Brain Injury (TBI)”
- As the number of cases continues to rise due to various contributing factors such as road accidents, sports-related injuries, and falls, particularly among the elderly population. Road traffic accidents remain one of the leading causes of TBI worldwide, with the growing number of vehicles on the road, reckless driving behaviours
- As lesões relacionadas com o desporto, especialmente em desportos de contacto como o futebol, o boxe e o rugby, aumentaram ainda mais o aumento de casos de TCE, com a crescente consciencialização sobre as complicações relacionadas com a concussão, o que levou à necessidade de soluções de tratamento avançadas.
Por exemplo,
- Em março de 2025, de acordo com o artigo publicado pela ScienceDirect, ocorreram 20,84 milhões de casos incidentes e 37,93 milhões de casos prevalentes de Traumatismo Cranioencefálico (TCE) na Europa, resultando em 5,48 milhões de Anos Vividos com Incapacidade (AVDs). O aumento da incidência de TCE aumenta a procura de tratamentos avançados, impulsionando os investimentos em diagnóstico, neurocirurgia e reabilitação, o que, em última análise, alimenta o crescimento do mercado de tratamento de TCE na Europa.
- Em outubro de 2024, de acordo com os dados publicados pelos Centros de Controlo e Prevenção de Doenças, em 2021, ocorreram 69.473 mortes relacionadas com TCE e, em 2020, ocorreram aproximadamente 214.110 hospitalizações. Isto equivale a mais de 586 hospitalizações e 190 mortes por dia, sendo os indivíduos com 75 ou mais anos e os homens os mais afetados. O aumento da incidência de TCE exige soluções de tratamento avançadas, impulsionando o crescimento do mercado europeu de tratamento do TCE
- Fatores como acidentes de viação, lesões desportivas e quedas — sobretudo entre os idosos — contribuem para o aumento de TCE. A crescente consciencialização e procura por tratamentos avançados, incluindo neurocirurgia, terapias medicamentosas e reabilitação, estão a alimentar a expansão do mercado e os avanços tecnológicos no tratamento do TCE.
Oportunidade
“Terapêuticas personalizadas e dirigidas em ascensão no traumatismo cranioencefálico (TCE)”
- O TCE é uma condição altamente variável influenciada pela gravidade, localização e fatores específicos do doente, tornando os tratamentos tradicionais menos eficazes. Os avanços na descoberta de biomarcadores, neuroimagiologia e modelação computacional ajudam a identificar padrões distintos de lesões, permitindo terapias mais direcionadas. A farmacogenómica melhora a seleção e a dosagem dos medicamentos, minimizando os efeitos secundários e maximizando a eficácia. Estratégias de reabilitação personalizadas, adaptadas às deficiências cognitivas e motoras, otimizam ainda mais a recuperação ao alinhar os tratamentos com as trajetórias de cura individuais
Por exemplo,
- Em fevereiro de 2022, de acordo com o NCBI, os investigadores descobriram fatores de risco genéticos, como os polimorfismos APOE4 e BDNF Val66Met, que afetam a recuperação do TCE. Ao focar-se nestas variações, os tratamentos personalizados podem reduzir os biomarcadores prejudiciais, aumentar a neuroproteção e melhorar a reabilitação. Esta abordagem adapta as terapêuticas às necessidades individuais, conduzindo, em última análise, a melhores resultados funcionais a longo prazo para os doentes com TCE
- Em fevereiro de 2024, o artigo MDPI TBI apresenta uma oportunidade significativa para melhorar os resultados dos doentes. Os avanços na descoberta de biomarcadores, farmacogenómica e neuroimagiologia permitem tratamentos de precisão adaptados a perfis de lesões individuais. As terapias emergentes, incluindo a neuroestimulação e os tratamentos com células estaminais, alargam ainda mais as possibilidades de intervenções eficazes e específicas para cada doente no tratamento do TCE.
- As terapias personalizadas e dirigidas apresentam uma abordagem transformadora para o tratamento da Lesão Cerebral Traumática (LCT) ao adaptar as intervenções aos perfis genéticos e moleculares individuais. Estas estratégias centram-se em biomarcadores e processos celulares específicos para reduzir os danos secundários e melhorar a recuperação. Ao otimizar o tratamento, as terapias personalizadas melhoram os resultados e promovem a recuperação funcional a longo prazo dos doentes com TCE.
Restrição/Desafio
“Dificuldades em ultrapassar a barreira hematoencefálica para o tratamento do TCE”
- Um desafio significativo no tratamento do Traumatismo Cranioencefálico (TCE) é a rotura da Barreira Hematoencefálica (BHE). Após um TCE, a barreira hematoencefálica (BHE) fica geralmente comprometida, permitindo a entrada de substâncias nocivas no cérebro, o que pode agravar a lesão e dificultar a recuperação. Isto cria dificuldades na administração eficaz de agentes terapêuticos, limitando o sucesso de muitos tratamentos concebidos para auxiliar na recuperação e proteger o tecido cerebral.
- Além disso, restaurar a integridade da Barreira Hematoencefálica (BHE) sem causar danos adicionais continua a ser um grande desafio. O desenvolvimento de sistemas de administração direcionados que possam contornar a barreira danificada sem introduzir mais riscos é crucial para melhorar os resultados do tratamento do TCE.
Por exemplo,
- Em janeiro de 2022, a Springer Nature Publishing Inc informou que a barreira hematoencefálica (BHE) restringe a administração de agentes terapêuticos ao cérebro. Mesmo quando a BHE é comprometida após uma lesão, muitos medicamentos, principalmente moléculas grandes, ainda têm dificuldade em penetrá-la, limitando a eficácia dos tratamentos e complicando as terapêuticas dirigidas.
- Em junho de 2024, a Nature Reviews Neurology informou que a disfunção da BHE pode persistir dias a anos após o TCE, contribuindo para complicações neurológicas a longo prazo. Esta disfunção está associada a edema, neuroinflamação e alterações nas redes neuronais, complicando as estratégias de tratamento e levando a comprometimentos cognitivos, depressão e epilepsia pós-traumática, desafiando assim a recuperação eficaz e as abordagens terapêuticas.
- A rutura da barreira hematoencefálica (BHE) representa um desafio significativo no tratamento do traumatismo cranioencefálico (TCE), uma vez que restringe a administração eficaz dos tratamentos e agrava os danos cerebrais. A disfunção persistente da BHE pode levar a complicações a longo prazo, incluindo inflamação, inchaço cerebral e distúrbios cognitivos. Embora abordagens como a terapia de eliminação de ERO sejam promissoras na melhoria da função cerebral, a natureza flutuante dos danos da BHE complica as estratégias terapêuticas. Para melhorar os resultados do TCE, existe uma necessidade crítica de sistemas inovadores de administração de medicamentos e de melhores métodos para monitorizar a integridade da BHE, permitindo tratamentos mais eficazes e reduzindo as deficiências neurológicas a longo prazo.
Âmbito de mercado de tratamento de lesão cerebral traumática
O mercado é segmentado com base no tratamento, idade do paciente, sexo, causa da lesão e utilizador final.
|
Segmentação |
Sub-segmentação |
|
Por tratamento |
|
|
Por idade do doente |
|
|
Por género |
|
|
Por causa de lesão |
|
|
Por utilizador final |
|
Análise regional do mercado de tratamento de lesões cerebrais traumáticas
“A Alemanha é o país dominante no mercado de tratamento de lesões cerebrais traumáticas”
- A Alemanha deverá liderar o mercado europeu de tratamento de lesões cerebrais traumáticas (LCT), devido à sua infraestrutura de saúde avançada e aos investimentos significativos em investigação e desenvolvimento médico.
- O país possui uma rede bem estabelecida de hospitais neurológicos especializados, centros de reabilitação e instituições de investigação focadas em lesões cerebrais e condições relacionadas.
- Além disso, as políticas de saúde da Alemanha apoiam cuidados de elevada qualidade ao doente, garantindo um diagnóstico precoce e opções de tratamento eficazes, o que reforça ainda mais a sua posição como líder de mercado no tratamento do TCE.
“ A Alemanha deverá registar a maior taxa de crescimento”
- Espera-se que a Alemanha experimente a maior Taxa de Crescimento Anual Composta (CAGR) neste mercado, impulsionada pela crescente consciencialização sobre as estratégias de gestão do TCE e pela adoção de modalidades de tratamento inovadoras.
- As crescentes campanhas de sensibilização pública e as iniciativas governamentais levaram a um melhor reconhecimento das lesões cerebrais traumáticas, resultando em intervenções mais precoces e melhores resultados para os doentes
- Além disso, a forte presença da Alemanha na biotecnologia e na indústria farmacêutica acelerou o desenvolvimento de tratamentos de ponta, como terapias regenerativas e medicamentos neuroprotetores, contribuindo para o rápido crescimento do mercado.
Participação no mercado de tratamento de lesão cerebral traumática
O cenário competitivo do mercado fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, presença na Europa, localizações e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento do produto, amplitude e abrangência do produto, domínio da aplicação. Os pontos de dados fornecidos acima estão apenas relacionados com o foco das empresas em relação ao mercado.
Os principais líderes de mercado que operam no mercado são:
- Pfizer, Lda. (EUA)
- Teva Pharmaceuticals US, Inc. (EUA)
- Fresenius SE & Co. KGaA (Fresenius Kabi AG) (Alemanha)
- Viatris Inc. (EUA)
- Amneal Produtos Farmacêuticos LLC. (NÓS)
- Laboratórios Dr. Reddy's Ltd. (Índia)
- Sun Pharmaceutical Industries, Inc. (Índia)
- Lupin (Índia)
- Hikma (Jordânia)
- Aurobindo Pharma US (Índia)
- UCI Médica (EUA)
- B. Braun Medical, Inc. (Alemanha)
- Alembic Pharmaceuticals Limited (Índia)
- Merz Therapeutics (Alemanha)
- Advacare (África do Sul)
- Maxzimaa (Índia)
- Jedux Parenteral Private Limited (Índia)
- Sagent Pharmaceuticals, Inc. (EUA)
- Swiss Pharma Nigeria Limited (Nigéria)
Últimos desenvolvimentos no mercado de tratamento de lesões cerebrais traumáticas
- Em fevereiro de 2024, a Viatris e a Idorsia estabeleceram uma importante colaboração de investigação e desenvolvimento na América do Norte para promover terapias inovadoras em diversas áreas terapêuticas. Esta parceria alavanca a experiência da Idorsia na descoberta de medicamentos e o alcance da Viatris na América do Norte, acelerando o desenvolvimento de tratamentos inovadores e expandindo os pipelines de ambas as empresas, reforçando o seu compromisso em satisfazer as necessidades médicas não satisfeitas em todo o mundo.
- Em fevereiro de 2021, a Fresenius Kabi expandiu as suas instalações na Áustria, reforçando as suas capacidades de produção e inovação em produtos farmacêuticos e tecnologias médicas. Esta expansão aumenta a eficiência do fabrico, garante um fornecimento constante de produtos de cuidados intensivos e apoia os avanços da investigação. Ao aumentar a capacidade e a excelência operacional, a empresa reforça a sua presença no mercado e satisfaz a crescente procura da América do Norte por soluções de saúde
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END-USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 TECHNOLOGY ROADMAP
4.4 VALUE CHAIN ANALYSIS
4.5 OPPUTUNITY MAP ANALYSIS
4.6 REIMBURSEMENT FRAMEWORK
4.7 COST ANALYSIS BREAKDOWN
4.8 PENETRATION AND GROWTH PROSPECT MAPPING: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
4.9 KEY PRICING STRATEGIES: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
4.1 MICRO AND MACRO-ECONOMIC FACTORS: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
5 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING INCIDENCE OF TRAUMATIC BRAIN INJURY (TBI)
6.1.2 GROWING ADOPTION OF MINIMALLY INVASIVE PROCEDURES IN TBI TREATMENT
6.1.3 TECHNOLOGICAL ADVANCEMENT FOR DIAGNOSIS OF TRAUMATIC BRAIN INJURIES (TBI)
6.1.4 ADVANCEMENTS IN NEUROPROTECTION AND PHARMACOTHERAPY FOR TBI TREATMENT
6.2 RESTRAINTS
6.2.1 SHORTAGE OF TRAINED NEUROLOGISTS AND NEUROSURGEONS
6.2.2 HIGH COST OF TRAUMATIC BRAIN INJURY (TBI) TREATMENT
6.3 OPPORTUNITIES
6.3.1 RISING PERSONALIZED & TARGETED THERAPIES IN TRAUMATIC BRAIN INJURY (TBI)
6.3.2 GROWING BRAIN STIMULATION TECHNIQUES IN TRAUMATIC BRAIN INJURY (TBI) TREATMENT
6.3.3 RISING ARTIFICIAL INTELLIGENCE (AI) APPLICATIONS IN DIAGNOSING TRAUMATIC BRAIN INJURY (TBI)
6.4 CHALLENGES
6.4.1 DIFFICULTIES IN OVERCOMING THE BLOOD-BRAIN BARRIER FOR TBI TREATMENT
6.4.2 ABSENCE OF STANDARDIZED TREATMENT PROTOCOLS IN TRAUMATIC BRAIN INJURY MANAGEMENT
7 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT
7.1 OVERVIEW
7.2 SURGERY
7.2.1 BRAIN BLEEDING TREATMENT
7.2.2 REHABILITATION
7.2.3 CLOTTED BLOOD REMOVAL
7.2.4 WINDOW OPENING IN SKULL
7.2.5 REPAIRING SKULL FRACTURES
7.3 IMMEDIATE EMERGENCY CARE
7.4 MEDICATIONS
7.4.1 DIURETICS
7.4.2 ANTI-SEIZURE DRUGS (ANTI-CONVULSANT)
7.4.3 ANALGESIC
7.4.4 COMA-INDUCING DRUGS
7.4.5 ANTI-DEPRESSANTS
7.4.6 ANTI-ANXIETY AGENT
7.4.7 ANTI-PSYCHOTICS
7.4.8 ANTI-COAGULANTS
7.4.9 OTHERS
7.4.9.1 Parenteral
7.4.9.2 Oral
7.4.9.3 Others
8 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE
8.1 OVERVIEW
8.2 CHILDREN
8.3 TEENAGER
8.4 ELDER
9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER
9.1 OVERVIEW
9.2 MALE
9.3 FEMALE
10 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, CAUSE OF INJURY
10.1 OVERVIEW
10.2 FALLS
10.3 MOTOR VEHICLE TRAFFIC
10.4 SPORTS
10.5 OTHERS
11 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 NEUROLOGY CLINICS
11.4 INDEPENDENT PHARMACIES
11.5 OTHERS
12 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 U.K.
12.1.3 FRANCE
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 NETHERLANDS
12.1.8 SWITZERLAND
12.1.9 TURKEY
12.1.10 BELGIUM
12.1.11 REST OF EUROPE
13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 PFIZER INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.2 TEVA PHARMACEUTICALS USA, INC.
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT/NEWS
15.3 FRESENIUS SE & CO. KGAA (FRESENIUS KABI AG)
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 VIATRIS INC.
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 AMNEAL PHARMACEUTICALS LLC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ADVACARE PHARMA
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 AUROBINDO PHARMA LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 ALEMBIC PHARMACEUTICALS LIMITED
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 B. BRAUN SE
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT UPDATES
15.1 DR. REDDY’S LABORATORIES LTD.
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PIPELINE PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 ICU MEDICAL, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT/NEWS
15.12 HIKMA PHARMACEUTICALS PLC
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PIPELINE PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 JEDUX PARENTERAL PRIVATE LIMITED
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 LUPIN
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MERZ THERAPEUTICS
15.15.1 COMPANY SNAPSHOT
15.15.2 PIPELINE PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 MAXZIMAA
15.16.1 COMPANY SNAPSHOT
15.16.2 PIPELINE PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 SUN PHARMACEUTICAL INDUSTRIES LTD.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 SWISS PHARMA NIGERIA LIMITED
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.19 SAGENT
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tabela
TABLE 1 NON-INVASIVE THERAPEUTIC APPROACHES’ EFFICIENCY IN DIFFERENT PHASES OF TBI
TABLE 2 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE IMMEDIATE EMERGENCY CARE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE CHILDREN IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE TEENAGER IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE ELDER IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE MALE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE FEMALE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE FALLS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE MOTOR VEHICLE TRAFFIC IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SPORTS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE OTHERS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE HOSPITALS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE NEUROLOGY CLINICS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE INDEPENDENT PHARMACIES IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE OTHERS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 35 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 36 GERMANY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GERMANY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 38 GERMANY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 39 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 40 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 41 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 42 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 43 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 44 U.K. SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 U.K. MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 U.K. MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 47 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 48 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 49 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 50 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 51 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 52 FRANCE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 FRANCE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 FRANCE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 55 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 56 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 57 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 58 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 59 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 60 ITALY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 ITALY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 ITALY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 63 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 64 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 65 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 66 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 67 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 68 SPAIN SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 SPAIN MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 SPAIN MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 71 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 72 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 73 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 74 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 75 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 76 RUSSIA SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 RUSSIA MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 RUSSIA MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 79 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 80 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 81 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 82 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 83 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 84 NETHERLANDS SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 NETHERLANDS MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 NETHERLANDS MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 87 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 88 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 89 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 90 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 91 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 92 SWITZERLAND SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 SWITZERLAND MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 SWITZERLAND MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 95 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 96 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 97 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 98 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 99 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 100 TURKEY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 TURKEY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 TURKEY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 103 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 104 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 105 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 106 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 107 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 108 BELGIUM SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 BELGIUM MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 BELGIUM MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 111 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 112 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 113 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 114 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 115 REST OF EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
Lista de Figura
FIGURE 1 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SEGMENTATION
FIGURE 2 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: MARKET END-USER COVERAGE GRID
FIGURE 9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SEGMENTATION
FIGURE 11 INCREASING INCIDENCE OF TRAUMATIC BRAIN INJURY (TBI) IS EXPECTED TO DRIVE THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 12 SURGERY SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 DROC ANALYSIS
FIGURE 16 TBI-RELATED DEATHS FROM 2018 TO 2024
FIGURE 17 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, 2024
FIGURE 18 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, 2025 TO 2032 (USD THOUSAND)
FIGURE 19 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, CAGR (2025- 2032)
FIGURE 20 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 21 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, 2024
FIGURE 22 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, 2025 TO 2032 (USD THOUSAND)
FIGURE 23 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, CAGR (2025- 2032)
FIGURE 24 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, LIFELINE CURVE
FIGURE 25 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, 2024
FIGURE 26 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER CAGR (2025-2032)
FIGURE 28 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, LIFELINE CURVE
FIGURE 29 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, 2024
FIGURE 30 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, CAGR (2025-2032)
FIGURE 32 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, LIFELINE CURVE
FIGURE 33 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, 2024
FIGURE 34 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY SHARE 2024 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.