Global Hiv Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
36.87 Billion
USD
59.66 Billion
2024
2032
USD
36.87 Billion
USD
59.66 Billion
2024
2032
| 2025 –2032 | |
| USD 36.87 Billion | |
| USD 59.66 Billion | |
|
|
|
|
Segmentação do mercado global do vírus da imunodeficiência humana (HIV), por diagnóstico (teste de antígeno/anticorpo, teste de anticorpos, testes de ácido nucleico (NATs), contagem de células T CD4, carga viral, resistência a medicamentos e outros), medicamentos ( inibidores de protease (IPs), medicamentos combinados multiclasse, inibidores de fusão (IF), inibidores nucleosídeos da transcriptase reversa (NRTIs) e outros), via de administração (oral, parenteral e outros), usuários finais (hospitais, assistência domiciliar, clínicas especializadas e outros) – Tendências do setor e previsão até 2032
Análise de Mercado do Vírus da Imunodeficiência Humana (HIV)
O mercado do vírus da imunodeficiência humana (HIV) tem apresentado crescimento substancial ao longo dos anos, impulsionado por avanços contínuos no tratamento e diagnóstico do HIV. O mercado é amplamente impulsionado pela crescente prevalência global do HIV, especialmente em países de baixa e média renda, juntamente com o desenvolvimento contínuo de terapias antirretrovirais (TARV) inovadoras. Os principais avanços no tratamento incluem a introdução de medicamentos injetáveis de ação prolongada, como o Lenacapavir (Sunlenca), que oferece aos pacientes opções de dosagem mais convenientes e melhora a adesão. Além disso, o lançamento de terapias combinadas, que combinam vários medicamentos para HIV em um único comprimido, aprimorou os regimes de tratamento, tornando-os mais eficazes e fáceis de administrar pelos pacientes.
O mercado também testemunha avanços em diagnósticos, com kits de detecção rápida e testes no local de atendimento, facilitando o diagnóstico precoce do HIV. Inovações como os kits de detecção rápida de ISTs da Mylab Discovery Solutions estão contribuindo para o esforço global de aumentar o diagnóstico precoce e melhorar o acesso a tratamentos. Além disso, colaborações entre grandes empresas farmacêuticas, como a Gilead Sciences e a Dr. Reddy's Laboratories, visam expandir o acesso a medicamentos que salvam vidas contra o HIV em mercados emergentes. De modo geral, esses avanços em tratamentos, diagnósticos e acessibilidade estão aprimorando significativamente a resposta global à epidemia de HIV.
Tamanho do mercado do vírus da imunodeficiência humana (HIV)
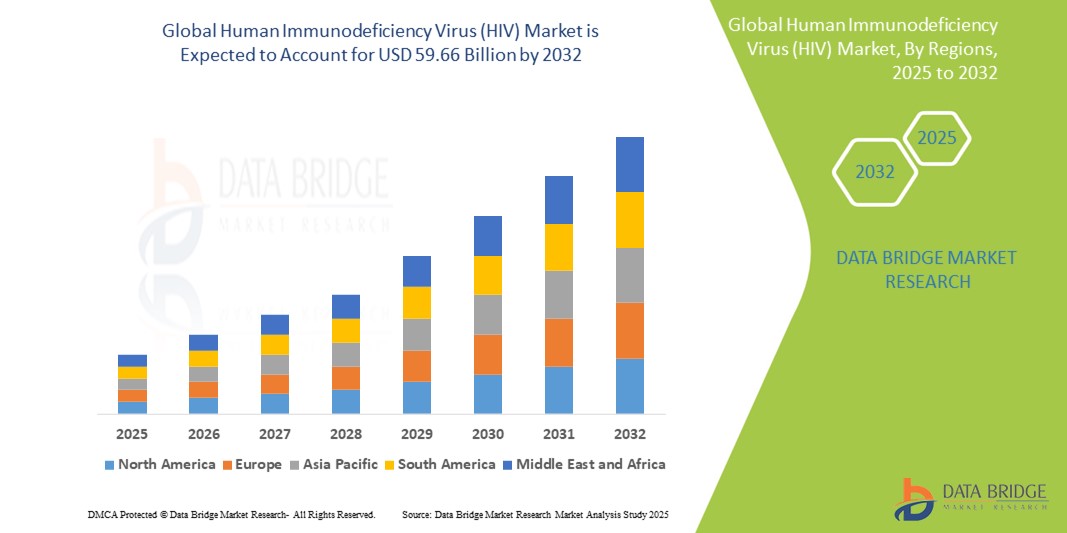
O tamanho do mercado global do vírus da imunodeficiência humana (HIV) foi avaliado em US$ 36,87 bilhões em 2024 e está projetado para atingir US$ 59,66 bilhões até 2032, com um CAGR de 6,20% durante o período previsto de 2025 a 2032. Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de pipeline, análise de preços e estrutura regulatória.
Tendências de mercado do vírus da imunodeficiência humana (HIV)
“ Crescente foco em terapias combinadas”
A key trend driving the human immunodeficiency virus (HIV) market is the shift towards long-acting injectable HIV treatments. These treatments, such as Lenacapavir (Sunlenca), allow for less frequent dosing compared to traditional daily oral medications, improving patient adherence and convenience. For instance, Gilead Sciences’ Sunlenca, approved by the European Commission in 2022, offers a groundbreaking solution for patients with multi-drug-resistant HIV, who previously had limited treatment options. This shift is particularly important in resource-limited settings, where access to daily oral treatments can be challenging. The growing adoption of long-acting injectables is enhancing the quality of life for individuals living with HIV, reducing the pill burden, and improving treatment outcomes. In addition, collaborations between companies such as Gilead Sciences and Dr. Reddy's Laboratories are expanding access to these advanced treatments, particularly in low- and middle-income countries, driving further growth in the market. This trend reflects a broader push towards more effective and convenient HIV care.
Report Scope and Human Immunodeficiency Virus (HIV) Market Segmentation
|
Attributes |
Human Immunodeficiency Virus (HIV) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
F. Hoffmann-La Roche Ltd. (Switzerland), Bristol-Myers Squibb Company (U.S.), AstraZeneca (U.K.), GSK plc (U.K.), Merck & Co., Inc. (U.S.), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Viatris Inc. (U.S.), Sanofi (France), Bayer AG (Germany), and Genentech, Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Human Immunodeficiency Virus (HIV) Market Definition
Human Immunodeficiency Virus (HIV) is a virus that attacks the immune system, specifically targeting CD4 cells (T cells), which are crucial in defending the body against infections. Over time, if untreated, HIV can weaken the immune system to the point where it can no longer effectively fight off infections and diseases, leading to Acquired Immunodeficiency Syndrome (AIDS).
Human Immunodeficiency Virus (HIV) Market Dynamics
Drivers
- Increase in Global HIV Cases
The escalating number of HIV cases worldwide emphasizes the urgent need for efficient treatment and prevention methods, driving the market’s growth. The World Health Organization (WHO) reports that approximately 39.9 million people were living with HIV by the end of 2023, with 1.4 million children under 15 years old affected. This concerning trend highlights the critical necessity for advancing antiretroviral therapy (ART) to better manage the virus and enhance the quality of life for patients. Awareness campaigns and educational efforts promoting preventive measures such as pre-exposure prophylaxis (PrEP) and regular testing are also pivotal. As healthcare systems address this growing crisis, the demand for innovative HIV solutions, including improved treatment options and comprehensive care, continues to drive market advancements and open opportunities for expansion.
- Advancements in Antiretroviral Therapies (ART)
The continuous innovations in antiretroviral therapies (ART) play a key role in propelling the HIV treatment market, improving both treatment effectiveness and patient adherence. Long-acting injectable formulations, for instance, enable patients to receive medication less frequently often monthly or quarterly making it easier for those who struggle with daily pill regimens. In addition, newer drug combinations are better equipped to target the virus, offering enhanced efficacy with fewer side effects, thus improving patient outcomes. As healthcare providers move towards more personalized care tailored to individual needs, the demand for advanced ART options is growing, ultimately spurring market growth.
Opportunities
- Rise of Long-Acting Therapies
The emergence of long-acting antiretroviral therapies (ART) offers a significant opportunity for advancing HIV treatment, particularly through the introduction of injectable medications and implantable devices. These therapies allow for less frequent dosing ranging from monthly to quarterly making it easier for patients to adhere to treatment plans, reducing the challenges of daily pill regimens. This flexibility is especially helpful for those struggling with forgetfulness or treatment fatigue. Long-acting therapies also help improve viral suppression and lower the transmission risk, which leads to better patient health outcomes. As these therapies gain traction, they expand the market by attracting a wider range of patients seeking manageable HIV treatment options.
- Innovations in Diagnostic Technologies
Innovative advancements in rapid testing and point-of-care diagnostics offer a promising market opportunity by enabling early detection and timely treatment of HIV. These advanced diagnostic tools provide quick results, often within minutes, allowing healthcare providers to intervene promptly and offer patients immediate insights into their health status. In areas with limited healthcare access, these tools are particularly beneficial, facilitating early diagnosis and improving HIV management. Furthermore, the ease of use and minimal training required for point-of-care tests encourage their adoption among healthcare professionals, making them a valuable asset in expanding HIV diagnostic capabilities.
Restraints/Challenges
- Lack of Personalized Treatment Options
The absence of personalized treatment options in antiretroviral therapy (ART) remains a significant challenge in HIV care. Although ART has led to better treatment outcomes overall, each patient's response to medication can differ due to genetic and physiological variations. This makes the standard approach ineffective for some, as they may face side effects or insufficient viral suppression. The limited availability of personalized medicine, such as pharmacogenomics to identify the most suitable therapies for individual patients, complicates treatment adherence and management, hindering the overall effectiveness of HIV care.
- Mental Health Barriers
Mental health challenges among individuals living with HIV present a significant barrier to effective treatment and improved health outcomes. Conditions such as depression and anxiety, often linked to the stigma of HIV, discrimination, and the emotional stress of living with a chronic illness, can reduce motivation to follow through with treatment regimens or attend medical appointments. These psychological obstacles worsen the challenges of treatment adherence and create a cycle of neglect. To address this, integrating mental health support and counseling into HIV care models is essential. Failure to do so impedes treatment effectiveness and strains healthcare systems, ultimately limiting market growth and the success of HIV treatment efforts.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Human Immunodeficiency Virus (HIV) Market Scope
The market is segmented on the basis of diagnostics, drugs, route of administration, and end-users. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Diagnostics
- Antigen/Antibody Test
- Antibody Test
- Nucleic Acid Tests (NATs)
- CD4T Cell Count
- Viral Load
- Drug Resistance
- Others
Drugs
- Protease Inhibitors (PIs)
- Multi-Class Combination Drugs
- Fusion Inhibitors (FI)
- Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
- Others
Route of Administration
- Oral
- Parenteral
- Others
End-Users
- Hospitals
- Homecare
- Specialty Clinics
- Others
Human Immunodeficiency Virus (HIV) Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, diagnostics, drugs, route of administration, and end-users as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific dominates the global HIV market, primarily due to the high prevalence of HIV/AIDS cases and the wide availability of generic drugs. In addition, substantial investments in the healthcare sector are fueling the market’s growth. The growing populations in India and China are expected to further drive the demand for HIV treatments in the coming years. With these combined factors, the Asia-Pacific market is poised for continued expansion and advancement in HIV treatment solutions.
North American HIV market has experienced fastest growth throughout the forecast period, driven by increased investments in healthcare and research and development. Rising patient awareness, coupled with the active involvement of major market players in creating innovative technologies and reformulating existing medications, has significantly contributed to this growth. These factors have collectively enhanced the HIV market landscape, fostering both innovation and greater accessibility. Consequently, the overall outlook for the HIV market in North America remains positive and promising.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Human Immunodeficiency Virus (HIV) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Human Immunodeficiency Virus (HIV) Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- AstraZeneca (U.K.)
- GSK plc (U.K.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Viatris Inc. (U.S.)
- Sanofi (France)
- Bayer AG (Germany)
- Genentech, Inc. (U.S.)
Latest Developments in Human Immunodeficiency Virus (HIV) Market
- In October 2024, Dr. Reddy's Laboratories announced its collaboration with Gilead Sciences for the manufacturing and commercialization of the HIV drug Lenacapavir in India and 120 other countries. The company has secured a royalty-free, non-exclusive voluntary licensing agreement with Gilead Sciences Ireland UC for the drug, marking a significant step in expanding access to HIV treatment in these regions
- In December 2023, Zydus Lifesciences received approval from the U.S. Food and Drug Administration (USFDA) to market a generic antiviral medication, Darunavir Tablets, for the treatment of HIV-1 infections. This approval opens new access to affordable HIV treatments in the U.S. market
- In August 2023, Aurobindo Pharma Ltd. launched its HIV triple combination product specifically designed for children living with HIV in low- and middle-income countries. With a voluntary pediatric dolutegravir license from ViiV Healthcare, Aurobindo can supply the product across 123 LMICs, including India
- In February 2023, Mylab Discovery Solutions, based in Pune, introduced three rapid kits for the early detection of sexually transmitted infections (STIs), including HIV, Hepatitis C, and Syphilis. These innovations aim to address the growing global burden of STIs, with the World Health Organization (WHO) projecting a daily caseload of one million STIs worldwide
- In August 2022, Gilead Sciences achieved a major milestone with the European Commission’s approval of Sunlenca (Lenacapavir), the first global regulatory approval for this novel treatment. Available in both injection and tablet forms, Sunlenca targets multi-drug-resistant HIV infections and is to be used alongside other antiretroviral therapies, offering new hope to patients facing limited treatment options
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.