Global Oncology Clinical Trial Monitor Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
12.70 Million
USD
19.50 Million
2022
2030
USD
12.70 Million
USD
19.50 Million
2022
2030
| 2023 –2030 | |
| USD 12.70 Million | |
| USD 19.50 Million | |
|
|
|
|
Global Oncology Clinical Trial Monitor Market, By Phase (Phase I, Phase II, Phase III, Phase IV), Study Design (Interventional, Observational, Expanded Access), Cancer Type (Lung Cancer, Breast Cancer, Leukemia, Prostate Cancer, Others) – Industry Trends and Forecast to 2030.
Oncology Clinical Trial Monitor Market Analysis and Size
The increasing number of people diagnosed with cancer is anticipated to increase multi-fold in the upcoming years. Although patients have increased significantly because of research in the oncology field, the number of cancer patients is expected to increase worldwide. Lung cancer is the top cause of cancer death, with an expected 1.8 million people diagnosed yearly worldwide. It is seen that the phase II segment dominated the oncology clinical trials market due to the increasing number of studies in phase II.
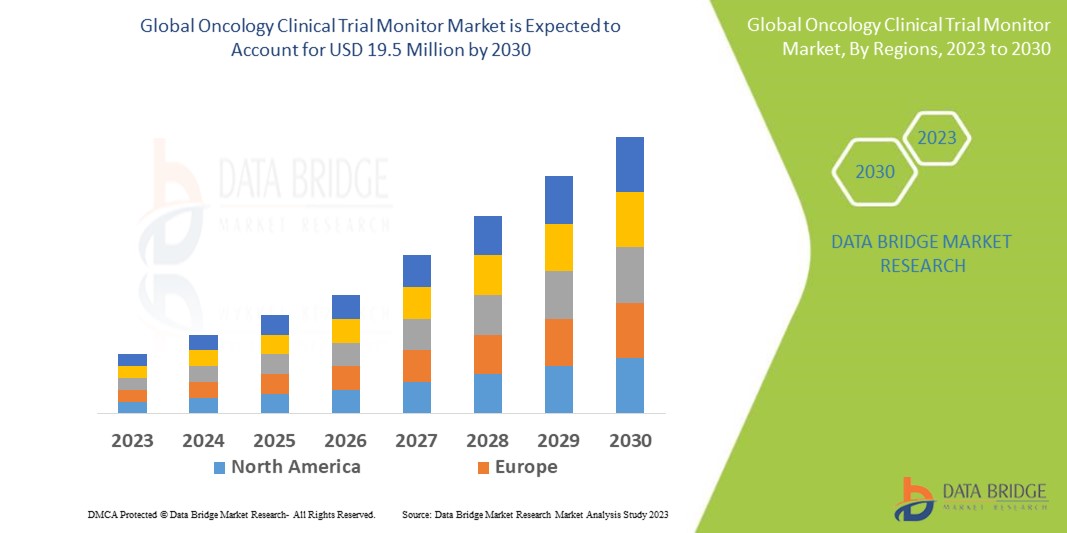
Data Bridge Market Research analyses that the oncology clinical trial monitor market, which was USD 12.70 in 2022, would rise to USD 19.5 million by 2030 and is expected to undergo a CAGR of 5.50% during the forecast period from 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Oncology Clinical Trial Monitor Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Phase (Phase I, Phase II, Phase III, Phase IV), Study Design (Interventional, Observational, Expanded Access), Cancer Type (Lung Cancer, Breast Cancer, Leukemia, Prostate Cancer, Others |
|
Countries Covered |
U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
IQVIA Inc (U.S.)., Charles River Laboratories (U.S)., ICON Plc (Ireland), Parexel International Corporation (U.S), WuXi AppTec (China), Syneos Health (U.S)., Labcorp Drug Development (U.S.)., Parexel International Corporation (U.S.), PPD Inc. (U.S)., and Medpace, Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Oncology clinical trials are the experiments performed under clinical research and follow a regulated protocol. These experiments are mainly performed to acquire the data associated with the safety and efficiency of the latest developed drugs. Clinical trial data is compulsory for drug approval and for it to be launched in the industry. These trials are conducted under different phases, which depend upon numerous factors.
Global Oncology Clinical Trial Monitor Market Dynamics
Drivers
- Increasing Number of Cancer Cases
There has been a growing number of cancer survivors living in the U.S., from 3.0 million in 1971 to 15.5 million in January 2016. About 73.0% of the cancer survival gains are sustainable to novel medicines. Several biopharmaceutical research firms are developing effective and better-tolerated treatments to meet the needs of patients. Around 85.0% of the oncology medicines in development are most likely to be first-in-class. In 2018, around 1,120 medicines and vaccines were in development by America's biopharmaceutical firms. Thus, this factor boosts market growth.
- Increasing Strategic Collaborations by Market Players
Increasing collaboration among many market players has led to market growth. For instance, IQVIA announced its collaboration with Cancer Researchers to advance the use of real-world evidence and enhance clinical research in oncology in 2019. Further, Acurian and Synexus, a part of PPD, launched SynexusPlus in 2018. SynexusPlus is a site solution for patient enrollment in different clinical studies. This initiative is projected to enhance clinical trial productivity. This is expected to impact the market growth positively.
Opportunities
- Increasing Demand for Phase II Trials
There is an increasing number of studies in phase II, which leads to market growth. Besides, since 2010 there has been a huge improvement in the total productivity of oncology clinical trials evaluated as success rates relative to the trial effort by 22%. Moreover, by the Medicines Healthcare Products Regulatory Agency (MHRA), between 2011 and 2016, the application for phase II and phase III trials increased by 5.5%. Thus, this factor boosts the market's growing rapidly.
Restraints/Challenges
- Lack of Trained Professionals
The trials in oncology are developing and emerging rapidly. Thus, not many people, but rather professionals, are well aware of the actual processing of the trials. These procedures require extremely high professionalism as a very sensitive process, and a slight mistake can lead to many complications. Thus, this factor leads to market hindrance.
This oncology clinical trial monitor market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the oncology clinical trial monitor market, contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Oncology Clinical Trial Monitor Market Scope
The oncology clinical trial monitor market is segmented on the basis of phase, study design and cancer type. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Study Design
- Interventional
- Observational
- Expanded Access
Cancer Type
- Lung Cancer
- Breast Cancer
- Leukemia
- Prostate Cancer
- Others
Oncology Clinical Trial Monitor Market Regional Analysis/Insights
The oncology clinical trial monitor market is analyzed and market size insights and trends are provided by phase, study design and cancer type as referenced above.
The countries covered in the oncology clinical trial monitor market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As a result of increased research and development, North America dominates the oncology clinical trial monitor market. Additionally, the increasing acceptance of modern technologies in clinical research and government support will further enhance the market growth in the region during the forecast period.
Asia-Pacific is expected to witness significant growth due to the increase in the obtainability of a large patient base allowing easy recruitment of candidates. Additionally, the increasing number of active investigators is further expected to boost the growth of the market in the region in the upcoming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The oncology clinical trial monitor market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for oncology clinical trial monitor market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the oncology clinical trial monitor market. The data is available for historic period 2011-2021.
Competitive Landscape and Oncology Clinical Trial Monitor Market Share Analysis
The oncology clinical trial monitor market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to oncology clinical trial monitor market.
Some of the major players operating in the oncology clinical trial monitor market are:
- IQVIA Inc (U.S.).
- Charles River Laboratories (U.S).
- ICON Plc (Ireland)
- Parexel International Corporation (U.S)
- WuXi AppTec (China)
- Syneos Health (U.S).
- Labcorp Drug Development (U.S.).
- Parexel International Corporation (U.S.)
- PPD Inc. (U.S).
- Medpace, Inc. (U.S.)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.