North America Cardiopulmonary Bypass Accessory Equipment Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
8.97 Billion
USD
14.08 Billion
2025
2033
USD
8.97 Billion
USD
14.08 Billion
2025
2033
| 2026 –2033 | |
| USD 8.97 Billion | |
| USD 14.08 Billion | |
|
|
|
|
Segmentação do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte, por produto (oxigenador, máquina de ECMO, bombas, cânulas, monitores de temperatura, trocadores de calor, filtros, grampos para tubos, hemoconcentradores, painéis de sistema, sensores e acessórios, controle de cardioplegia, reservatórios, detectores de bolhas, misturadores eletrônicos de gases, oclusores venosos elétricos, grampos para linhas venosas e acessórios), operação (manual, elétrica e a bateria), aplicação (cirurgia cardíaca, oxigenadores para cirurgia cardíaca, tratamento de insuficiência respiratória aguda, câncer de pulmão, transplante e outros), faixa etária (adulto, geriátrico e pediátrico), usuário final (hospitais, centros cardíacos, instituições de pesquisa e acadêmicas, centros cirúrgicos ambulatoriais e outros), canal de distribuição (licitação direta, distribuidores terceirizados e varejo) - Tendências e previsões do setor até 2033.
Tamanho do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
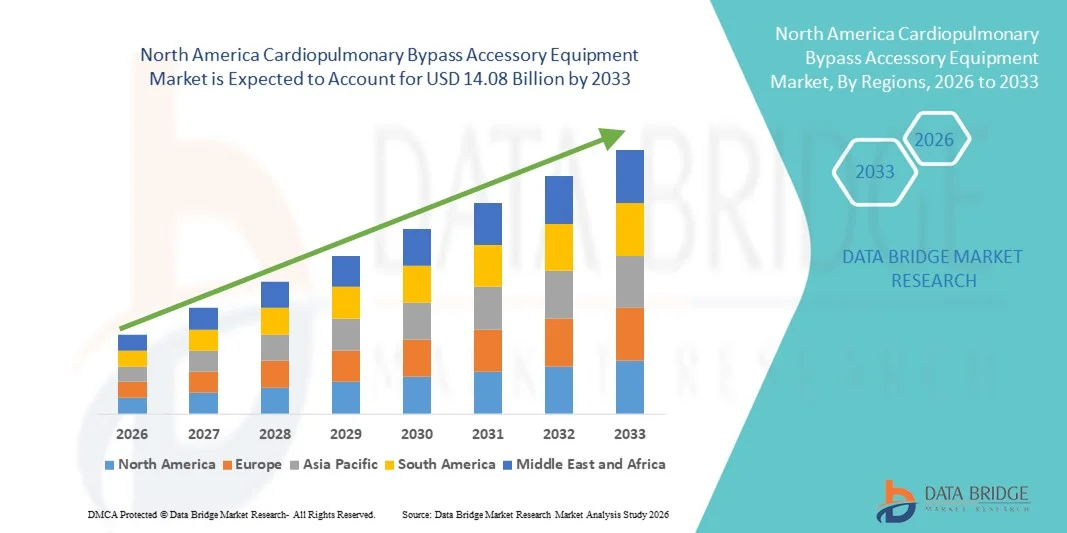
- O mercado de equipamentos acessórios para circulação extracorpórea na América do Norte foi avaliado em US$ 8,97 bilhões em 2025 e deverá atingir US$ 14,08 bilhões até 2033 , com uma taxa de crescimento anual composta (CAGR) de 5,8% durante o período de previsão.
- O crescimento do mercado é impulsionado principalmente pela crescente prevalência de doenças cardiovasculares, pelo aumento do número de cirurgias cardíacas e pelos avanços tecnológicos contínuos em sistemas de circulação e perfusão extracorpórea, que aprimoram a eficiência dos procedimentos e a segurança do paciente.
- Além disso, a infraestrutura de saúde robusta, os elevados gastos com saúde, as políticas de reembolso favoráveis e a adoção de dispositivos acessórios avançados, como oxigenadores, cânulas, filtros e trocadores de calor, estão impulsionando a demanda em hospitais, centros cardíacos e ambientes cirúrgicos ambulatoriais, tornando os equipamentos acessórios para circulação extracorpórea a escolha preferencial para o tratamento cardíaco moderno.
Análise do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
- Os equipamentos acessórios para circulação extracorpórea, incluindo oxigenadores, cânulas, filtros e trocadores de calor, são componentes essenciais em cirurgias cardíacas e procedimentos de circulação extracorpórea em hospitais e centros cirúrgicos ambulatoriais, devido à sua capacidade de aumentar a segurança do paciente, a eficiência do procedimento e os resultados clínicos.
- A crescente demanda por esses dispositivos é impulsionada principalmente pela prevalência cada vez maior de doenças cardiovasculares, pelo aumento do número de cirurgias cardíacas e pelos avanços tecnológicos contínuos em sistemas de perfusão que melhoram a confiabilidade, a precisão e a integração com equipamentos cirúrgicos modernos.
- Os EUA dominaram o mercado de equipamentos acessórios para circulação extracorpórea, com a maior participação de mercado, de 79,5% em 2025. Esse crescimento foi impulsionado por uma infraestrutura de saúde avançada, altos gastos com saúde, políticas de reembolso favoráveis e forte presença de empresas líderes do setor. A adoção significativa desses equipamentos em centros cardíacos e hospitais foi impulsionada por inovações em perfusão minimamente invasiva e sistemas acessórios modulares.
- Prevê-se que o Canadá seja o país com o crescimento mais rápido no mercado de equipamentos acessórios para circulação extracorpórea durante o período de previsão, devido ao aumento dos investimentos em infraestrutura de saúde, à expansão das instalações de atendimento cardíaco e à crescente conscientização sobre tecnologias cirúrgicas avançadas.
- O segmento de oxigenadores dominou o mercado de equipamentos acessórios para circulação extracorpórea, com uma participação de 38,7% em 2025, devido ao seu papel crucial na manutenção de trocas gasosas eficazes e à preferência consolidada entre perfusionistas e cirurgiões cardíacos.
Escopo do relatório e segmentação do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte.
|
Atributos |
Principais informações sobre o mercado de equipamentos acessórios para circulação extracorpórea na América do Norte |
|
Segmentos abrangidos |
|
|
Países abrangidos |
América do Norte
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além das informações sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais players, os relatórios de mercado elaborados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de projetos em desenvolvimento, análise de preços e estrutura regulatória. |
Tendências do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
Avanços tecnológicos e integração com sistemas de perfusão
- Uma tendência significativa e crescente no mercado de equipamentos acessórios para circulação extracorpórea na América do Norte é a integração de tecnologias avançadas de perfusão e sistemas cirúrgicos modulares, aprimorando a eficiência operacional e a segurança do paciente durante procedimentos cardíacos.
- Por exemplo, oxigenadores e trocadores de calor estão sendo cada vez mais projetados para se integrarem perfeitamente com dispositivos de perfusão automatizados, reduzindo erros humanos e otimizando os fluxos de trabalho cirúrgicos.
- Os avanços em cânulas e filtros com sensores permitem o monitoramento em tempo real do fluxo sanguíneo, da pressão e da oxigenação, possibilitando aos perfusionistas ajustes rápidos e precisos. Por exemplo, algumas bombas centrífugas modernas incluem algoritmos com auxílio de inteligência artificial para otimizar o fluxo com base na hemodinâmica específica de cada paciente.
- Essa tendência em direção a equipamentos acessórios mais inteligentes, interconectados e automatizados está remodelando fundamentalmente as expectativas clínicas para a cirurgia cardíaca. Por exemplo, empresas como a Medtronic e a Terumo estão desenvolvendo oxigenadores e filtros compatíveis com sistemas automatizados de monitoramento e controle adaptativo de fluxo.
- A adoção de dispositivos de circulação extracorpórea avançados e integrados como esses está crescendo rapidamente em hospitais e centros cardíacos dos EUA, à medida que a equipe clínica prioriza cada vez mais a precisão, a segurança e a integração perfeita com os equipamentos cirúrgicos existentes.
- Os recursos de conectividade aprimorados também permitem suporte e solução de problemas remotos para equipamentos de perfusão, possibilitando que os hospitais acessem orientação especializada durante cirurgias complexas e reduzindo a probabilidade de atrasos nos procedimentos.
Dinâmica do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
Motorista
Aumento do volume de cirurgias cardíacas e da prevalência da doença.
- A crescente prevalência de doenças cardiovasculares, aliada ao número cada vez maior de cirurgias cardíacas, é um fator significativo para o aumento da demanda por equipamentos acessórios para circulação extracorpórea.
- Por exemplo, em março de 2025, a Terumo Cardiovascular anunciou o lançamento de um oxigenador avançado projetado para melhorar a eficiência da troca gasosa durante procedimentos de bypass prolongados, demonstrando o foco da indústria na inovação.
- À medida que hospitais e centros cardíacos buscam aprimorar a segurança do paciente e os resultados cirúrgicos, acessórios como filtros, cânulas e oxigenadores fornecem suporte crucial para manter a perfusão e a hemodinâmica estáveis.
- Além disso, a crescente adoção de cirurgias cardíacas minimamente invasivas e complexas está ampliando a necessidade de sistemas acessórios de bypass precisos, modulares e compatíveis.
- A demanda por equipamentos que oferecem monitoramento em tempo real, integração confiável com dispositivos de perfusão e compatibilidade com diversas configurações cirúrgicas está impulsionando sua adoção nos EUA, à medida que a equipe clínica prioriza cada vez mais a eficiência, a segurança e a padronização dos procedimentos.
- Por exemplo, a crescente conscientização entre os cirurgiões sobre complicações relacionadas à perfusão está impulsionando investimentos em acessórios avançados de monitoramento para melhorar os resultados dos pacientes.
- A colaboração tecnológica entre fabricantes de equipamentos e hospitais também está impulsionando a inovação em acessórios para circulação extracorpórea, levando a uma adoção mais rápida de dispositivos de última geração.
Restrição/Desafio
Custos elevados e requisitos de conformidade regulamentar
- O custo relativamente elevado dos dispositivos acessórios avançados para circulação extracorpórea, aliado aos rigorosos requisitos regulamentares, representa um desafio para uma maior penetração no mercado.
- Por exemplo, hospitais ou clínicas menores podem adiar a atualização para os oxigenadores ou filtros mais recentes devido a restrições orçamentárias, limitando a adoção em ambientes com custos controlados.
- A conformidade com as normas da FDA e da ISO, juntamente com a necessidade de validação extensiva e testes clínicos, aumenta a complexidade e o tempo de lançamento de novos dispositivos no mercado. Por exemplo, novos designs de cânulas devem passar por rigorosos testes de desempenho e biocompatibilidade antes da aprovação.
- Embora as inovações melhorem os resultados clínicos, o investimento inicial necessário para equipamentos avançados de perfusão pode dificultar a aquisição em certos hospitais dos EUA, principalmente em instalações menores ou regionais.
- Superar esses desafios por meio de estratégias de otimização de custos, processos regulatórios simplificados e educação clínica direcionada será vital para o crescimento sustentado e a adoção mais ampla de equipamentos acessórios para circulação extracorpórea.
- Por exemplo, os hospitais estão explorando modelos de leasing ou aquisição conjunta de acessórios de perfusão para reduzir os custos iniciais e melhorar o acesso às tecnologias mais recentes.
- O aumento da concorrência entre os fabricantes também está impulsionando a inovação e pressionando os preços, o que ajuda mais hospitais a adotarem sistemas avançados de acessórios para bypass, apesar das restrições orçamentárias.
Escopo do mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
O mercado é segmentado com base em produto, operação, aplicação, idade, usuário final e canal de distribuição.
- Por produto
Com base no produto, o mercado é segmentado em oxigenadores, máquinas de ECMO, bombas, cânulas, monitores de temperatura, trocadores de calor, filtros, pinças para tubos, hemoconcentradores, painéis de sistema, sensores e acessórios, controle de cardioplegia, reservatórios, detectores de bolhas, misturadores eletrônicos de gases, oclusores venosos elétricos, pinças para linhas venosas e acessórios. O segmento de oxigenadores dominou o mercado com a maior participação de receita, de 38,7% em 2025, impulsionado por seu papel crucial na manutenção de trocas gasosas eficazes durante procedimentos de circulação extracorpórea. Os oxigenadores são amplamente utilizados em cirurgias cardíacas, tratamentos de insuficiência respiratória aguda e transplantes devido à sua confiabilidade, facilidade de uso e compatibilidade com sistemas de perfusão modulares. Hospitais e centros cardíacos preferem oxigenadores por seu comprovado perfil de segurança e desempenho consistente durante procedimentos de alto risco. Avanços tecnológicos, como sensores integrados e otimização de fluxo assistida por IA, reforçam ainda mais a dominância desse segmento. Além disso, os oxigenadores são essenciais para minimizar complicações e garantir a sobrevivência do paciente durante cirurgias complexas, tornando-se um elemento fundamental dos equipamentos acessórios de bypass.
O segmento de máquinas de ECMO deverá apresentar o crescimento mais rápido entre 2026 e 2033, impulsionado pelo aumento de casos de insuficiência respiratória aguda e aplicações em terapia intensiva. As máquinas de ECMO oferecem suporte vital para pacientes com condições cardiopulmonares graves e são cada vez mais utilizadas em unidades de terapia intensiva pediátrica e geriátrica. Os avanços tecnológicos, incluindo sistemas de ECMO portáteis e modulares, estão ampliando sua adoção tanto em hospitais terciários quanto em centros cardíacos especializados. A crescente conscientização sobre a terapia com ECMO entre os médicos, aliada a um melhor treinamento e infraestrutura, está impulsionando o crescimento. A pandemia de COVID-19 e outras emergências respiratórias também destacaram a importância dos sistemas de ECMO, acelerando a demanda. O segmento se beneficia da inovação contínua em monitoramento automatizado, eficiência de oxigenação e controle de perfusão.
- Por Operação
Com base no modo de operação, o mercado é segmentado em operado manualmente, operado eletricamente e operado por bateria. O segmento de operação elétrica dominou o mercado em 2025 devido à sua precisão, capacidade de automação e integração com sistemas modernos de perfusão cirúrgica. Os hospitais preferem cada vez mais bombas, cânulas e oxigenadores operados eletricamente para garantir um fluxo sanguíneo consistente e reduzir a intervenção manual durante procedimentos complexos. Este segmento permite monitoramento em tempo real, controle adaptativo do fluxo e alertas para perfusionistas, melhorando a segurança do paciente e a eficiência cirúrgica. A integração da operação elétrica com painéis digitais e consoles de perfusão aumenta a compatibilidade com os sistemas de informação hospitalares. Cirurgiões e perfusionistas valorizam a confiabilidade, a reprodutibilidade e a redução da carga de trabalho oferecidas pelos dispositivos operados eletricamente. As constantes atualizações tecnológicas, como os ajustes de fluxo assistidos por inteligência artificial, reforçam ainda mais essa dominância.
Prevê-se que o segmento de equipamentos operados por bateria apresente o crescimento mais rápido entre 2026 e 2033, impulsionado pela necessidade de portabilidade em centros cirúrgicos ambulatoriais e em cuidados cardíacos de emergência. Bombas, oxigenadores e sistemas de ECMO operados por bateria oferecem flexibilidade durante o transporte, procedimentos fora do ambiente hospitalar e em locais com restrições de energia. A maior autonomia da bateria, os designs leves e as configurações modulares estão impulsionando a adoção em ambientes de terapia intensiva. A crescente demanda em aplicações pediátricas e geriátricas, onde a mobilidade dos equipamentos é crucial, alimenta ainda mais o crescimento. Os fabricantes estão focando em recursos de confiabilidade e segurança, incluindo alimentação de reserva e regulação automática do fluxo. O segmento está ganhando popularidade em instalações de saúde remotas e de menor porte que buscam soluções de bypass de alta qualidade sem infraestrutura elétrica permanente.
- Por meio de aplicação
Com base na aplicação, o mercado é segmentado em cirurgia cardíaca, oxigenadores para cirurgia cardíaca, tratamento de insuficiência respiratória aguda, câncer de pulmão, transplante e outros. O segmento de Cirurgia Cardíaca dominou o mercado com a maior participação na receita em 2025, impulsionado pelo volume consistentemente alto de procedimentos cardíacos abertos e minimamente invasivos em hospitais dos EUA. Acessórios para circulação extracorpórea, como oxigenadores, cânulas, filtros e trocadores de calor, são essenciais para manter a estabilidade do paciente durante a cirurgia. Os cirurgiões dependem de dispositivos de alta precisão e validados para perfusão, reduzindo o risco de complicações. A disponibilidade de sistemas avançados de monitoramento integrados a painéis cirúrgicos fortalece ainda mais esse segmento. Os hospitais priorizam equipamentos padronizados e confiáveis que garantam a eficiência do procedimento e a segurança do paciente. Inovações tecnológicas, incluindo otimização de fluxo assistida por IA e alertas de segurança automatizados, aprimoram os resultados cirúrgicos.
O segmento de tratamento da insuficiência respiratória aguda deverá apresentar o crescimento mais rápido entre 2026 e 2033, devido ao aumento da prevalência de doenças respiratórias e internações em UTI. Máquinas de ECMO, oxigenadores e acessórios de perfusão associados estão sendo cada vez mais utilizados no tratamento de pacientes críticos. A crescente conscientização dos profissionais de saúde, o aprimoramento do treinamento em terapia com ECMO e a maior portabilidade dos dispositivos impulsionam essa adoção. Monitoramento avançado, integração com ventiladores e maior eficiência na oxigenação são fatores-chave para o crescimento. Pacientes pediátricos e geriátricos se beneficiam dessas aplicações, contribuindo para a expansão do segmento. Hospitais e centros cardíacos estão investindo em equipamentos especializados para lidar com emergências respiratórias agudas de forma eficiente.
- Por idade
Com base na idade, o mercado é segmentado em adultos, geriátricos e pediátricos. O segmento de adultos dominou o mercado em 2025 devido à alta prevalência de doenças cardiovasculares e ao fato de a maioria das cirurgias cardíacas ser realizada em pacientes adultos. Equipamentos acessórios para circulação extracorpórea, como oxigenadores, bombas e cânulas, são amplamente utilizados em cirurgias cardíacas em adultos, transplantes e procedimentos de terapia intensiva. Os hospitais priorizam dispositivos que ofereçam desempenho confiável, minimizem complicações e se integrem a sistemas de monitoramento de perfusão. Avanços tecnológicos, como monitoramento assistido por inteligência artificial e alertas automatizados, aumentam a segurança e a eficiência dos procedimentos de circulação extracorpórea em adultos. A grande quantidade de pacientes adultos nos EUA, combinada com a infraestrutura hospitalar avançada, sustenta a dominância do segmento. Sistemas de perfusão padronizados para adultos também garantem resultados consistentes, contribuindo para a manutenção da liderança de mercado.
O segmento pediátrico deverá apresentar o crescimento mais rápido entre 2026 e 2033, devido à crescente conscientização sobre cardiopatias congênitas e à necessidade de equipamentos de circulação extracorpórea especializados para crianças. Oxigenadores pediátricos, máquinas de ECMO e bombas são projetados para volumes sanguíneos menores e hemodinâmica sensível. O aumento de cirurgias cardíacas pediátricas e de cuidados intensivos neonatais impulsiona a demanda. Os fabricantes estão lançando dispositivos modulares, leves e com sensores integrados para aplicações pediátricas. O crescimento é ainda impulsionado pelos investimentos hospitalares em instalações especializadas em cardiologia pediátrica. Aprimoramentos tecnológicos e programas de treinamento para médicos que lidam com casos pediátricos contribuem para a rápida adoção desses produtos.
- Por usuário final
Com base no usuário final, o mercado é segmentado em hospitais, centros cardíacos, instituições de pesquisa e acadêmicas, centros cirúrgicos ambulatoriais e outros. O segmento de hospitais dominou o mercado em 2025, impulsionado pelo alto volume de cirurgias cardíacas, transplantes e tratamentos em UTI que requerem acessórios para circulação extracorpórea. Os hospitais preferem oxigenadores, bombas e sistemas de monitoramento avançados para um gerenciamento de perfusão confiável e eficiente. A integração com painéis de controle hospitalares e sistemas de monitoramento de pacientes aprimora a eficiência do fluxo de trabalho e a segurança do paciente. A disponibilidade de equipes cirúrgicas especializadas e perfusionistas favorece a adoção. Os avanços tecnológicos, incluindo monitoramento automatizado e alertas com auxílio de inteligência artificial, são amplamente implementados em hospitais. A grande infraestrutura e a capacidade de investimento dos hospitais reforçam ainda mais sua posição dominante.
O segmento de Centros Cirúrgicos Ambulatoriais deverá apresentar o crescimento mais rápido entre 2026 e 2033, devido ao aumento de procedimentos cardíacos ambulatoriais e cirurgias minimamente invasivas. Sistemas de ECMO portáteis, bombas operadas por bateria e oxigenadores compactos estão sendo cada vez mais adotados nesses ambientes. Os centros ambulatoriais se beneficiam de dispositivos leves, modulares e fáceis de usar. A relação custo-benefício e a flexibilidade no gerenciamento do fluxo de trabalho são fatores-chave para a adoção. A crescente preferência dos pacientes por procedimentos ambulatoriais está impulsionando a demanda. Os fabricantes estão desenvolvendo dispositivos personalizados para implantação rápida e atendimento cardíaco móvel, o que contribui para o crescimento do segmento.
- Por canal de distribuição
Com base no canal de distribuição, o mercado é segmentado em licitação direta, distribuidores terceirizados e vendas no varejo. O segmento de Licitação Direta dominou o mercado em 2025, impulsionado pela preferência de hospitais e centros cardíacos pela aquisição direta dos fabricantes para garantir a autenticidade do produto, a conformidade com as normas e a entrega pontual de equipamentos essenciais para circulação extracorpórea. As licitações diretas permitem personalização, compras em grande volume e acesso a suporte técnico para dispositivos complexos. Os hospitais se beneficiam de programas de treinamento do fabricante, serviço pós-venda e suporte à instalação. Contratos de longo prazo por meio de acordos de licitação direta garantem o fornecimento consistente de oxigenadores, bombas e cânulas. A conformidade com as normas regulatórias é facilitada por meio de negociações diretas. A confiabilidade, a confiança e o suporte do fabricante reforçam a dominância desse canal.
O segmento de distribuidores terceirizados deverá apresentar o crescimento mais rápido entre 2026 e 2033, devido ao aumento da demanda em hospitais menores, centros ambulatoriais e instalações de saúde remotas. Os distribuidores oferecem acesso a uma ampla gama de produtos, preços competitivos e opções de entrega flexíveis. Hospitais com orçamentos de compras limitados se beneficiam das redes de distribuição. A distribuição terceirizada também facilita a rápida adoção de novas tecnologias, conectando fabricantes e usuários finais. Os serviços de treinamento e suporte oferecidos pelos distribuidores aumentam ainda mais a penetração no mercado. A crescente demanda por cadeias de suprimentos eficientes e econômicas impulsiona o crescimento do segmento.
Análise Regional do Mercado de Equipamentos Acessórios para Circulação Extracorpórea na América do Norte
- Os EUA dominaram o mercado de equipamentos acessórios para circulação extracorpórea, com a maior participação de mercado, de 79,5% em 2025, impulsionados por uma infraestrutura de saúde avançada, altos gastos com saúde, políticas de reembolso favoráveis e forte presença de empresas líderes do setor.
- Nos Estados Unidos, hospitais e centros cardíacos priorizam a segurança do paciente, a eficiência dos procedimentos e a perfusão confiável, o que leva à ampla adoção de oxigenadores, bombas, cânulas e outros acessórios essenciais para circulação extracorpórea.
- Essa forte presença no mercado é ainda mais reforçada pelos elevados gastos com saúde, políticas de reembolso favoráveis, inovação tecnológica em sistemas de perfusão e pela presença de fabricantes líderes de dispositivos médicos, consolidando os EUA como o principal mercado de equipamentos acessórios para circulação extracorpórea na América do Norte.
Análise do Mercado de Equipamentos Acessórios para Circulação Extracorpórea nos EUA
O mercado de equipamentos acessórios para circulação extracorpórea nos EUA detinha a maior participação de mercado na América do Norte em 2025, com 79,5% da receita, impulsionado pelo uso generalizado de sistemas de circulação extracorpórea em hospitais terciários e pela crescente prevalência de doenças cardíacas. Hospitais e centros cardíacos estão priorizando equipamentos avançados de perfusão, como oxigenadores, bombas e sistemas de monitoramento, para melhorar a segurança cirúrgica e os resultados. A adoção de procedimentos minimamente invasivos, tecnologias de perfusão automatizadas e soluções de monitoramento com inteligência artificial está impulsionando ainda mais o crescimento do mercado. Uma infraestrutura de saúde robusta, políticas de reembolso favoráveis e a presença de importantes fabricantes de dispositivos médicos reforçam a liderança do mercado americano. Além disso, o alto volume de procedimentos cirúrgicos cardíacos e tratamentos de terapia intensiva sustenta uma forte demanda por dispositivos acessórios para circulação extracorpórea.
Análise do Mercado de Equipamentos Acessórios para Circulação Extracorpórea no Canadá
O mercado canadense de equipamentos acessórios para circulação extracorpórea deverá crescer a uma taxa composta de crescimento anual (CAGR) notável durante o período de previsão, impulsionado pelo aumento dos investimentos em saúde e pela expansão das instalações de atendimento cardíaco em todo o país. Os hospitais canadenses estão adotando cada vez mais equipamentos avançados, como máquinas de ECMO, oxigenadores e acessórios de perfusão com sensores integrados, para aumentar a segurança do paciente e a eficiência dos procedimentos. Iniciativas governamentais que apoiam a modernização da saúde e a atualização da infraestrutura cirúrgica estão contribuindo para a adoção de soluções de circulação extracorpórea de última geração. Além disso, a crescente conscientização sobre doenças cardiovasculares e a demanda por monitoramento de alta precisão em cirurgias cardíacas estão impulsionando o crescimento do mercado. Programas de treinamento aprimorados para perfusionistas e clínicos também contribuem para uma maior adoção de acessórios sofisticados para circulação extracorpórea no Canadá.
Análise do Mercado de Equipamentos Acessórios para Circulação Extracorpórea no México
O mercado mexicano de equipamentos acessórios para circulação extracorpórea está preparado para uma expansão gradual durante o período de previsão, à medida que as reformas na saúde e o desenvolvimento da infraestrutura melhoram a qualidade dos serviços de cardiologia. O aumento dos investimentos em tecnologias médicas, juntamente com a crescente conscientização sobre as opções de tratamento de doenças cardiovasculares, está impulsionando a demanda por equipamentos acessórios para circulação extracorpórea em hospitais terciários e centros cardíacos especializados. Os esforços dos provedores de saúde públicos e privados para modernizar as instalações cirúrgicas e adotar sistemas de perfusão avançados estão contribuindo para o crescimento do mercado. No entanto, o crescimento do mercado é atualmente mais modesto em comparação com os EUA e o Canadá, devido ao volume de procedimentos e aos gastos com saúde comparativamente menores. Espera-se que a melhoria contínua dos marcos regulatórios e o acesso a equipamentos avançados impulsionem ainda mais o crescimento do mercado.
Participação de mercado de equipamentos acessórios para circulação extracorpórea na América do Norte
O setor de equipamentos acessórios para circulação extracorpórea na América do Norte é liderado principalmente por empresas consolidadas, incluindo:
- Medtronic (Irlanda)
- LivaNova PLC (Reino Unido)
- Braile Biomédica (Brasil)
- Teleflex Incorporated (EUA)
- Getinge AB (Suécia)
- Corporação Terumo (Japão)
- Surge Cardiovascular (EUA)
- Abbott (EUA)
- Edwards Lifesciences Corporation (EUA)
- (Suíça)
- NIPRO Corporation (Japão)
- XENIOS AG (Alemanha)
- Advin Health Care (Índia)
- Boston Scientific Corporation (EUA)
- APC Cardiovascular Ltda. (Reino Unido)
- Corporação Científica MicroPort (China)
- Alung Technologies, Inc. (EUA)
- Coração de Berlim (Alemanha)
- Jarvik Heart, Inc. (EUA)
- Narang Medical Limited (Índia)
- Technowood International Pte. Ltd. (Singapura)
Quais são os desenvolvimentos recentes no mercado de equipamentos acessórios para circulação extracorpórea na América do Norte?
- Em setembro de 2024, a Medtronic plc lançou o Sistema de Oxigenação por Membrana Extracorpórea (ECMO) VitalFlow™ nos Estados Unidos, apresentando uma solução configurável de ECMO em um único sistema, projetada para integrar o atendimento à beira do leito e o transporte intra-hospitalar com uma interface amigável e suporte integrado a dados de desempenho, expandindo ainda mais seu portfólio de dispositivos para cirurgia cardíaca e perfusão após a aquisição da MC3 Cardiopulmonary.
- Em agosto de 2024, a Inspira™ Technologies anunciou que seu sistema INSPIRA™ ART100 recebeu a aprovação 510(k) da FDA para procedimentos de circulação extracorpórea, marcando um importante marco regulatório para este dispositivo de circulação e oxigenação extracorpórea e possibilitando seu lançamento comercial no mercado de saúde dos EUA. A aprovação confirma a segurança e a eficácia do ART100 para suporte temporário de oxigenação e circulação sanguínea.
- Em maio de 2024, a Terumo Cardiovascular anunciou que a Food and Drug Administration (FDA) dos EUA concedeu a aprovação 510(k) ao seu Sistema de Monitoramento CDI OneView™, uma plataforma modular avançada que fornece até 22 parâmetros essenciais do paciente durante a cirurgia de bypass cardiopulmonar, oferecendo às equipes de perfusão maior visibilidade e configurabilidade para promover resultados clínicos mais seguros durante procedimentos complexos.
- Em maio de 2024, a FDA (Administração de Alimentos e Medicamentos dos EUA) emitiu um comunicado de segurança aconselhando os profissionais de saúde a deixarem de usar certos dispositivos cardiovasculares da Getinge/Maquet, incluindo o sistema Cardiohelp e os componentes de bypass associados, devido a preocupações contínuas com a qualidade e a segurança, apesar de múltiplos recolhimentos voluntários. Isso levou os hospitais a considerarem soluções alternativas de perfusão e bypass.
- Em março de 2023, a LivaNova recebeu a aprovação 510(k) da FDA para sua Máquina Coração-Pulmão Essenz (HLM) e Monitor de Paciente Essenz, marcando o lançamento nos EUA do Sistema de Perfusão Essenz, projetado para aprimorar os fluxos de trabalho clínicos com controle individualizado da bomba, verificações automatizadas de sensores e posicionamento otimizado de materiais descartáveis para dar suporte a procedimentos de circulação extracorpórea personalizados.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.