North America Dermatology Treatment Devices Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
1.80 Billion
USD
3.60 Billion
2024
2032
USD
1.80 Billion
USD
3.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.80 Billion | |
| USD 3.60 Billion | |
|
|
|
|
North America Dermatology Treatment Devices Market Segmentation, by type (Push Button Safety Lancet, Pressure Activated Safety Lancet, Side Button Safety Lancet), by application (Blood Glucose Testing, Hemoglobin Testing, Cholesterol Testing, Coagulation Testing), by end user (Hospitals & Clinics, Diagnostic Centers and Pathology Laboratories, Home Diagnostics, Others)- Industry Trends and Forecast to 2032
North America Dermatology Treatment Devices Market Size
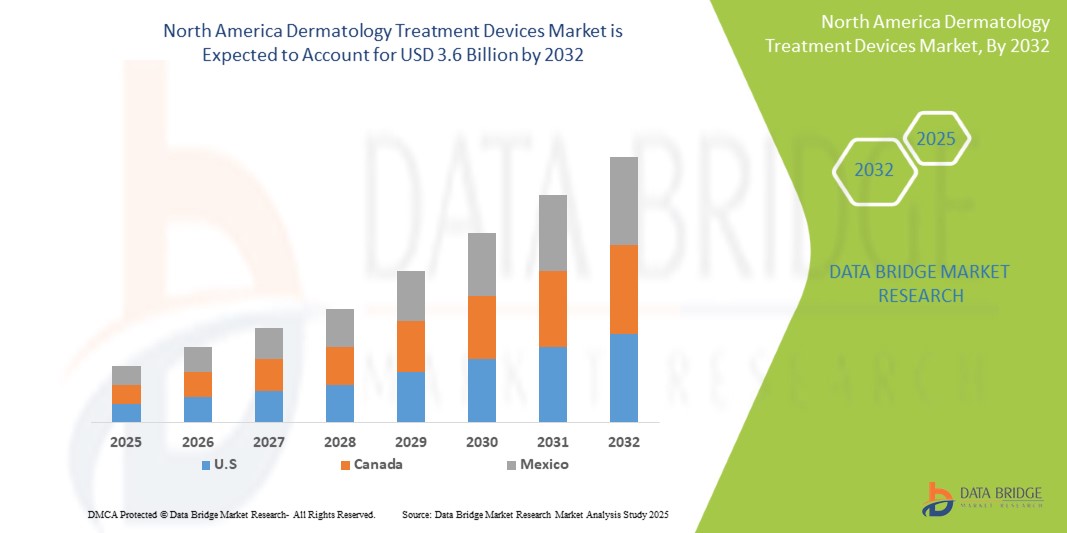
- The North America Dermatology Treatment Devices Market was valued atUSD1.8 Billionin 2024and is expected to reachUSD3.6 Billionby 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 8.9%,primarily driven by the increasing prevalence of chronic diseases
- Key drivers of the North America Dermatology Treatment Devices Market include the rising prevalence of chronic diseases like diabetes, growing awareness of needle-stick injuries, and the demand for safer and more efficient blood sampling devices.
North America Dermatology Treatment Devices Market Analysis
- The increasing focus on minimizing needle-stick injuries in healthcare settings is driving the adoption of safety lancets as a safer alternative for blood sampling.
- The rise in chronic diseases, especially diabetes, is fueling the demand for blood glucose monitoring devices, including safety lancets, for at-home and clinical use.
- For instance, Innovations in lancet design, such as push-button mechanisms and pressure-activated features, are enhancing user comfort and safety, further contributing to market growth..
- Increasing awareness regarding safe and hygienic blood sampling techniques in both medical and home care settings is promoting the use of safety lancets.
- As healthcare infrastructure improves, especially in developing regions, the demand for advanced medical devices, including safety lancets, is expected to rise significantly.
Report Scope and North America Dermatology Treatment Devices Market Segmentation
|
Attributes |
North America Dermatology Treatment Devices Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Dermatology Treatment Devices Market Trends
“integration of smart technology and connectivity features”
- Enhanced Patient Monitoring and Data Collection: Smart safety lancets are equipped with features like integrated biosensors and Bluetooth connectivity, enabling real-time data transmission to digital health platforms. This facilitates continuous monitoring of blood parameters and supports proactive healthcare interventions.
- Improved Patient Compliance and Engagement: By connecting to mobile applications, these devices provide users with reminders, usage statistics, and health insights, thereby encouraging regular testing and adherence to treatment plans.
- Support for Remote Healthcare Services: The integration of smart technology aligns with the growing demand for telemedicine and home healthcare services, allowing healthcare providers to remotely monitor patients' health status and make informed decisions without the need for in-person visits.
North America Dermatology Treatment Devices Market Dynamics
Driver
“rising prevalence of chronic diseases”
- Increased Demand for Regular Blood Sampling: Chronic diseases require frequent blood tests to monitor parameters like glucose levels, cholesterol, and hemoglobin, leading to a higher need for safe and efficient blood sampling devices.
- Enhanced Patient Safety and Compliance: Safety lancets are designed to minimize the risk of needlestick injuries and cross-contamination, encouraging patients to adhere to regular testing schedules.
- Alignment with Healthcare Regulations: The adoption of safety lancets supports healthcare facilities in complying with stringent safety standards and infection control protocols, promoting their widespread use in clinical settings.
Opportunity
“development of sustainable and eco-friendly safety lancets”
- Environmental Impact Reduction: With increasing global emphasis on sustainability, manufacturers are innovating safety lancets using biodegradable materials and recyclable packaging to minimize medical waste.
- Regulatory Compliance and Market Demand: Adhering to environmental regulations and responding to consumer demand for eco-conscious products can enhance brand reputation and market share.
- Alignment with Healthcare Sustainability Goals: Integrating sustainable practices in product development aligns with the broader healthcare industry's shift towards greener solutions, potentially opening new market segments and partnerships.
Restraint/Challenge
“high cost of advanced lancet technologies”
- Increased Production Costs: Advanced safety lancets often incorporate features like adjustable penetration depths and ergonomic designs, which enhance user comfort and safety but also increase manufacturing expenses.
- Limited Accessibility in Developing Regions: The elevated costs of these advanced devices can make them less accessible in developing countries, where healthcare budgets are constrained, and cost-effective alternatives are preferred.
North America Dermatology Treatment Devices Market Scope
The market is segmented on the basis application, type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Type |
|
|
By End User |
|
North America Dermatology Treatment Devices Market Regional Analysis
“North America is the Dominant Region in the North America Dermatology Treatment Devices Market”
- High Prevalence of Chronic Diseases: The region exhibits a significant incidence of chronic conditions like diabetes, cardiovascular diseases, and cancer. For instance, in the United States, approximately 6 out of 10 adults have at least one chronic disease, necessitating regular blood sampling for management and monitoring.
- Robust Healthcare Infrastructure and Reimbursement Policies: North America boasts advanced healthcare facilities and favorable reimbursement policies, facilitating the adoption of safety lancets. In Canada, initiatives such as the Disability Tax Credit for insulin users underscore the region's commitment to supporting individuals with chronic conditions
- Technological Advancements and Market Growth: The region is witnessing continuous innovation in safety lancet technologies, including features like adjustable penetration depths and ergonomic designs. This progression, coupled with a substantial patient base and increasing healthcare expenditure, positions North America to account for a significant share of the market's growth
“Asia-Pacific is Projected to Register the Highest Growth Rate in the North America Dermatology Treatment Devices Market”
- High Prevalence of Diabetes: Over 60% of the Asian population is living with diabetes, with China and India accounting for nearly half of this number. The Western Pacific region alone has more than 138.2 million people with diabetes, a number expected to rise to 201.8 million by 2035. This surge in diabetic cases necessitates frequent blood sampling, driving demand for safety lancets.
- Government Initiatives Promoting Screening: Countries like India and China are implementing large-scale health initiatives to combat chronic diseases. For instance, India's Union Health Ministry launched a program in 2023 aiming to screen 75 million people for hypertension and diabetes by 2025. Similarly, China's 'Healthy China 2030' initiative focuses on integrated diabetes management across urban and rural areas, further increasing the need for diagnostic tools like safety lancets.
- Advancements in Healthcare Infrastructure: Rapid urbanization and improvements in healthcare services across Asia-Pacific are enhancing access to medical diagnostics. The rising incidence of infectious diseases, such as malaria, dengue, and chikungunya, which require diagnostic testing, is also contributing to the increased demand for safety lancets in the region.
North America Dermatology Treatment Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd
- Becton, Dickinson and Company (BD)
- Terumo Medical Corporation
- Bayer AG
- HTL-STREFA S.A.
- Sarstedt AG & Co. KG
- Improve Medical Technology Co. Ltd
- Ypsomed AG
- Greiner Bio-One International GmbH
- Owen Mumford Ltd
- Smiths Medical
- Nipro Corporation
- Cardinal Health, Inc.
- Arkray Inc.
- Medline Industries, Inc.
Latest Developments in North America Dermatology Treatment Devices Market
- In 2021, FUJIFILM Corporation opened NURA, a medical screening center focusing on cancer screening in Bangalore, India. This medical screening center is operated by FUJIFILM DKH LLP (FUJIFILM DKH) and Dr. Kutty’s Healthcare (DKH). FUJIFILM DKH LLP (FUJIFILM DKH) is a joint venture of FUJIFILM and Dr. Kutty’s Healthcare (DKH), which runs hospitals and screening centers in India and the Middle East.
- In 2023, Astellas Pharma announced that it has entered into an agreement with Roche Diabetes Care Japan Co., Ltd. for the development and commercialization of Roche Diabetes Care’s world-renowned Accu-Chek Guide Me blood glucose monitoring system with advanced accuracy as a combined medical product with BlueStar.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.