North America Mycoplasma Testing In Clinical Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
136.90 Million
USD
240.55 Million
2022
2030
USD
136.90 Million
USD
240.55 Million
2022
2030
| 2023 –2030 | |
| USD 136.90 Million | |
| USD 240.55 Million | |
|
|
|
North America Mycoplasma Testing in Clinical Market, By Products (Kits and Reagents, Instruments, Services), Technique (Microbial Culture Techniques/Direct Assay, Polymerase Chain Reaction, ELISA, DNA Staining/Indirect Assay, Enzymatic Methods), Disease Area (Respiratory, Urogenital, Gastrointestinal, Musculoskeletal, Cardiovascular, Others), End User (Diagnostic Laboratories, Hospitals) – Industry Trends and Forecast to 2030.
North AmericaMycoplasma Testing in Clinical Market Analysis and Size
It has been witnessed that COPD is a primary cause of disability and death in the U.S. More than 12.5 million people have been diagnosed with COPD, but millions of the people have the disease without even knowing it. The number of deaths from pulmonary disease, respiratory illnesses, and tuberculosis has increased, and these figures are estimated to increase further in the upcoming years, which will increase the growth of the market.
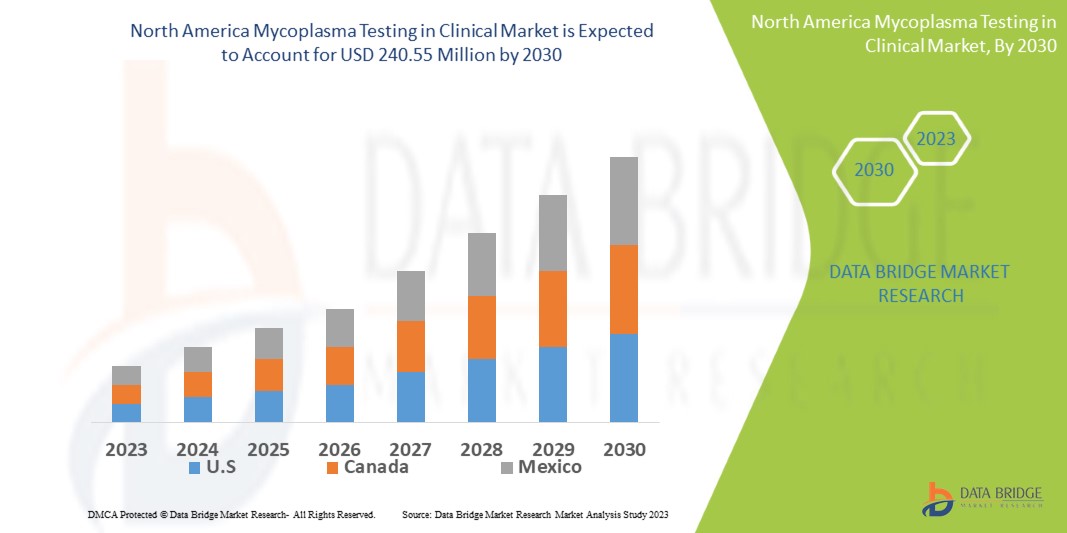
Data Bridge Market Research analyses a growth rate in the mycoplasma testing in clinical market in the forecast period 2023-2030. The expected CAGR of mycoplasma testing in clinical market is tend to be around 7.30% in the mentioned forecast period. The market is valued at USD 136.90 million in 2022, and it would grow upto USD 240.55 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
North AmericaMycoplasma Testing in Clinical Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Products (Kits and Reagents, Instruments, Services), Technique (Microbial Culture Techniques/Direct Assay, Polymerase Chain Reaction, ELISA, DNA Staining/Indirect Assay, Enzymatic Methods), Disease Area (Respiratory, Urogenital, Gastrointestinal, Musculoskeletal, Cardiovascular, Others), End User (Diagnostic Laboratories, Hospitals |
|
Countries Covered |
U.S., Canada and Mexico |
|
Market Players Covered |
AB ANALITICA s.r.l. (Italy), BIOMÉRIEUX (France), ELITechGroup (France), Liofilchem S.r.l. (Italy), Agilent Technologies, Inc. (U.S.), PromoCell GmbH (Germany), F. Hoffmann-La Roche Ltd (Switzerland), OSANG Healthcare (South Korea), Sacace Biotechnologies Srl (Italy), Lonza (Switzerland), Merck KGaA (Germany), Seegene Inc. (South Korea), Clongen Laboratories, LLC (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Charles River Laboratories (U.S.), Bionique Testing Laboratories LLC (U.S.) and ZEAKON Diagnostics (India) |
|
Market Opportunities |
|
Market Definition
Mycoplasma testing is widely used to identify an active or recent mycoplasma infection, which is a kind of bacterial infection. Testing may be done in a number of ways depending on which one is suspected, as there are different types of mycoplasma infections such as Mycoplasma is M. pneumoniae, which causes upper respiratory infections and can be further tested with a blood sample.
North America Mycoplasma Testing in Clinical Dynamics
Drivers
- Growing Adoption of PCR
PCR has been growing adoption in the last few years for quick detection and fast results. For instance, the CFX96 Touch Real-Time PCR Detection System from Bio-Rad Laboratories is based on PCR. This system is a versatile and accurate PCR detection system with six channels that consist of cutting-edge optical technique and exact temperature control. Consequently, both multiplex and singleplex reactions can be detected using this trustworthy and sensitive method. Therefore, this factor increases the growth of the market.
- Launch of Newer Technologies
The growing adoption of new technologies for drug research and development and advancements in cell culture technology boosts the growing need for mycoplasma tests. For instance, bioMérieux SA launched a molecular biology test termed BIOFIRE MYCOPLASMA test in July 2020, to detect mycoplasma in biotherapeutics. This is an innovative and easy to use test that can increase the detection of mycoplasma in biopharmaceutical products. This factor improves the market growth.
Opportunities
- Higher Demand of Kits and Reagents
The market is seem to rise due to the increased demand for these products in mycoplasma detection, and their high cost. Numerous market players have developed mycoplasma detection kits particularly to the needs of researchers and biologic manufacturers, which is boosting segment growth. Instruments are expected to experience major growth during the forecast period because of the increasing availability of automated equipment for the detection of mycoplasma. Thus, this factor created much opportunity for the market growth.
- Increase Incidence of Respiratory Tract Infections
The incidence of respiratory tract infections reported by numerous studies ranges from 20 to 30% in the U.S. In adults, upper respiratory tract infection were caused mostly by S. pneumoniae (21.62%) and LRTIs by human rhinovirus (26.47%). In the elderly group, URTIs were caused mostly by influenza A virus (33.33%), human rhinovirus (33.33%), and LRTIs by S. pneumoniae (50%) and human adenovirus (50%). Thus, this factor increases the growth of the market.
Restraints/Challenges
- Inadequate Reimbursement Policies
Even though several testing products were launched, but not all the products have been launched at a cheap price. An inadequate reimbursement scenario is estimated to restrict the use of mycoplasma testing and reduce the growth of the market during the forecast period 2023-2030. Thus, this hampers the market growth.
This mycoplasma testing in clinical market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the mycoplasma testing in clinical market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
North AmericaMycoplasma Testing in Clinical Market Scope
The mycoplasma testing in clinical market is segmented on the basis of products, technique, disease area and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Products
- Kits and Reagents
- Instruments
- Services
Technique
- Microbial Culture Techniques/Direct Assay
- Polymerase Chain Reaction
- ELISA
- DNA Staining/Indirect Assay
- Enzymatic Methods
Disease Area
- Respiratory
- Urogenital
- Gastrointestinal
- Musculoskeletal
- Cardiovascular
- Others
End-User
- Diagnostic Laboratories
- Hospitals
Mycoplasma Testing in Clinical Market Regional Analysis/Insights
The mycoplasma testing in clinical market is analyzed and market size insights and trends are provided by products, technique, disease area and end user as referenced above.
The major countries covered in the mycoplasma testing in clinical market report are U.S., Canada and Mexico.
The U.S. is leading the market due to increased healthcare funding. In North America region, kits & reagents segment is anticipated to increase the market growth in the forecast period of 2020 to 2027 due to growing mycoplasma testing in clinical field and research development for identification of mycoplasma cell culture contamination.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of Middle East and Africa brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The mycoplasma testing in clinical market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for placental stem cells (PSCS) market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the placental stem cells (PSCS) market. The data is available for historic period 2011-2021.
Competitive Landscape and North AmericaMycoplasma Testing in Clinical Market Share Analysis
The mycoplasma testing in clinical market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Middle East and Africa presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to mycoplasma testing in clinical market
Key players operating in the mycoplasma testing in clinical market include:
- AB ANALITICA s.r.l. (Italy)
- BIOMÉRIEUX (France)
- ELITechGroup (France)
- Liofilchem S.r.l. (Italy)
- Agilent Technologies, Inc. (U.S.)
- PromoCell GmbH (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- OSANG Healthcare (South Korea)
- Sacace Biotechnologies Srl (Italy)
- Lonza (Switzerland)
- Merck KGaA (Germany)
- Seegene Inc. (South Korea)
- Clongen Laboratories, LLC (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Charles River Laboratories (U.S.)
- Bionique Testing Laboratories LLC (U.S.)
- ZEAKON Diagnostics (India)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.