Asia Pacific Internal Neuromodulation Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
1.51 Billion
USD
7.07 Billion
2025
2033
USD
1.51 Billion
USD
7.07 Billion
2025
2033
| 2026 –2033 | |
| USD 1.51 Billion | |
| USD 7.07 Billion | |
|
|
|
|
Asia-Pacific Internal Neuromodulation Devices Market Size
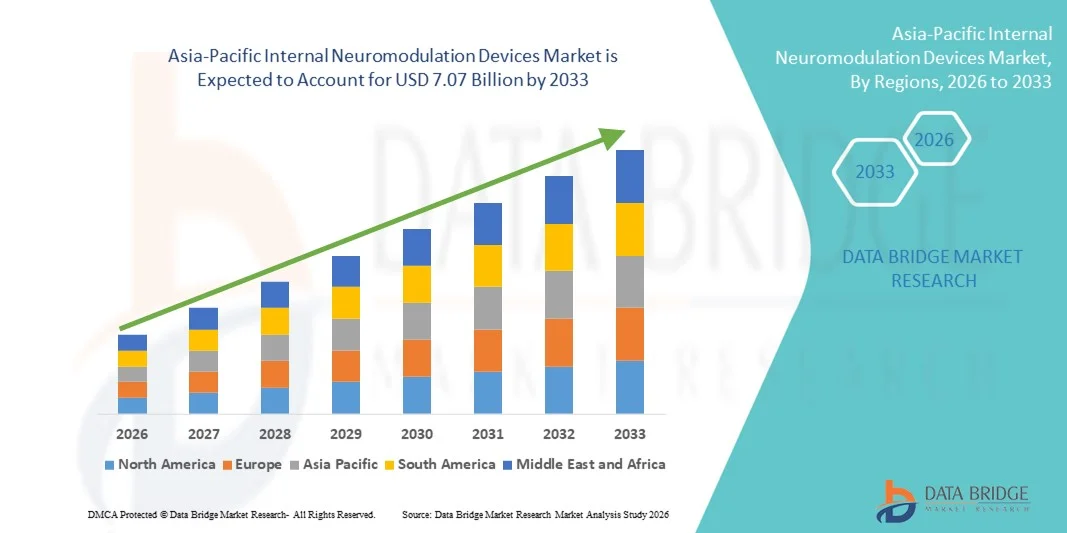
- The Asia-Pacific internal neuromodulation devices market size was valued at USD 1.51 billion in 2025 and is expected to reach USD 7.07 billion by 2033, at a CAGR of 21.3% during the forecast period
- The market’s growth trajectory is largely fueled by rising healthcare expenditure, increased prevalence of neurological and chronic pain disorders, and the adoption of advanced implantable devices such as spinal cord stimulators and deep brain stimulators. Continued technological innovation and supportive regulatory developments across major APAC countries are further accelerating demand
- Furthermore, expanding awareness about neuromodulation therapies, coupled with ageing populations in countries such as China, India, and Japan, is creating greater demand for surgical and implantable solutions. Strategic partnerships, local manufacturing initiatives, and improved healthcare access across emerging APAC markets are also positioning internal neuromodulation as a key treatment approach boosting overall industry growth throughout the forecast period
Asia-Pacific Internal Neuromodulation Devices Market Analysis
- Internal neuromodulation devices, including spinal cord stimulators, deep brain stimulators, and sacral nerve stimulators, are increasingly critical in managing chronic pain, neurological disorders, and movement disorders in both hospital and outpatient settings due to their therapeutic precision, implantable design, and programmable features
- The rising adoption of internal neuromodulation devices is primarily driven by growing prevalence of chronic pain and neurological conditions, increasing awareness among patients and healthcare providers, and advancements in device technology such as miniaturization, wireless programming, and MRI-compatible implants
- Japan dominated the Asia-Pacific internal neuromodulation devices market with the largest revenue share of 30.2% in 2025, characterized by advanced healthcare infrastructure, strong reimbursement policies, and early adoption of innovative neuromodulation therapies, with hospitals and specialty clinics increasingly implementing these devices for improved patient outcomes
- India is expected to be the fastest-growing country in the Asia-Pacific internal neuromodulation devices market during the forecast period due to rising healthcare expenditure, expanding neurological care facilities, increasing patient awareness, and improving access to advanced treatment options
- Spinal cord stimulators segment dominated the Asia-Pacific internal neuromodulation devices market with the largest market share of 45.9% in 2025, driven by their established efficacy in treating chronic pain, ease of implantation, and widespread adoption across hospitals and pain management centers
Report Scope and Asia-Pacific Internal Neuromodulation Devices Market Segmentation
|
Attributes |
Asia-Pacific Internal Neuromodulation Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Asia-Pacific Internal Neuromodulation Devices Market Trends
Advancements in Wireless and MRI-Compatible Devices
- A significant and accelerating trend in the Asia-Pacific internal neuromodulation devices market is the development of wireless-enabled and MRI-compatible implantable devices, enhancing patient comfort, clinical flexibility, and therapy personalization
- For instance, the latest spinal cord stimulators now allow wireless programming and remote adjustments, enabling clinicians to fine-tune stimulation without repeated hospital visits. Similarly, deep brain stimulators with MRI-safe components provide safer imaging for patients with neurological disorders
- Wireless connectivity in these devices enables remote monitoring, real-time therapy adjustments, and data-driven treatment optimization, while MRI-compatible designs allow patients to safely undergo diagnostic imaging without device removal. For instance, modern sacral nerve stimulators track patient activity patterns to adjust stimulation parameters automatically
- The integration of these advanced devices with hospital IT and patient management platforms facilitates centralized monitoring and therapy management, improving patient adherence and outcomes across neurology and pain management departments
- This trend toward more precise, patient-friendly, and technologically integrated neuromodulation systems is reshaping therapy delivery, with companies such as Boston Scientific developing MRI-safe and wireless spinal cord stimulators for improved patient convenience and treatment efficiency
- The demand for wireless and MRI-compatible internal neuromodulation devices is growing rapidly across hospitals and specialty clinics in China, Japan, and India, as clinicians prioritize improved clinical outcomes and patient-centric therapy management
- Another trend is the adoption of closed-loop neuromodulation systems that automatically adjust stimulation in response to patient physiological signals, improving therapy efficacy and reducing clinician intervention
Asia-Pacific Internal Neuromodulation Devices Market Dynamics
Driver
Rising Prevalence of Chronic Pain and Neurological Disorders
- The increasing prevalence of chronic pain, movement disorders, and neurological conditions in Asia-Pacific countries, coupled with expanding access to advanced healthcare facilities, is a significant driver for the growing adoption of internal neuromodulation devices
- For instance, in 2025, Medtronic launched a next-generation spinal cord stimulator in Japan, enabling improved therapy customization for chronic pain patients. Such innovations by key companies are expected to drive market growth during the forecast period
- As patient awareness of advanced neuromodulation therapies increases, devices offering programmable stimulation, remote monitoring, and therapy tracking provide a compelling alternative to conventional treatments for chronic pain and neurological disorders
- Furthermore, the growing expansion of hospitals and specialty neurology and pain management centers is making internal neuromodulation devices an integral part of treatment protocols, enabling seamless integration with multidisciplinary care plans
- The clinical advantages of minimally invasive implantation, remote programmability, and precise therapy targeting are key factors propelling adoption in China, India, and South Korea, with the trend toward patient-centric therapy management further contributing to market growth
- Increasing government initiatives and reimbursement programs for chronic pain management and neurological care in countries such as Japan and South Korea are facilitating wider adoption of neuromodulation devices
- Collaborations between device manufacturers and hospitals for clinical research and post-market studies are also driving confidence in therapy efficacy, accelerating market uptake across the region
Restraint/Challenge
High Device Costs and Regulatory Compliance Hurdles
- The high cost of internal neuromodulation devices and implantation procedures, along with complex regulatory approval requirements, poses a significant challenge to broader market penetration in developing countries of Asia-Pacific
- For instance, reports indicate that price-sensitive patients in India and Southeast Asia may delay adoption of advanced spinal cord stimulators despite clinical need due to upfront costs and limited reimbursement coverage
- Ensuring regulatory compliance, securing approvals across multiple countries, and addressing patient affordability through insurance and cost-reduction strategies is crucial for expanding market reach. For instance, companies such as Abbott emphasize clinical efficacy and regulatory certifications to reassure hospitals and patients
- While device prices are gradually decreasing and lower-cost options are emerging, the perceived premium for advanced neuromodulation systems can still hinder adoption, especially for patients in rural or underfunded healthcare settings
- Overcoming these challenges through cost-effective device development, expanded reimbursement coverage, and clinician and patient education on therapy benefits will be vital for sustained growth across Asia-Pacific countries such as China, India, and Japan
- Limited trained clinical specialists in some APAC countries for device implantation and post-operative management can slow adoption rates and affect patient outcomes
- Challenges in maintaining long-term device performance and managing potential complications such as infection or device migration may also deter clinicians and patients from early adoption of neuromodulation therapies
Asia-Pacific Internal Neuromodulation Devices Market Scope
The market is segmented on the basis of product type, lead type, biomaterial, application, and end-user.
- By Product Type
On the basis of product type, the Asia-Pacific internal neuromodulation devices market is segmented into spinal cord stimulators, deep brain stimulators, vagus nerve stimulators, sacral nerve stimulators, and gastric nerve stimulators. The spinal cord stimulator segment dominated the market in 2025 with the largest revenue share of 45.9%, driven by its established efficacy in treating chronic pain and failed back surgery syndrome. Hospitals and specialty clinics often prioritize spinal cord stimulators due to their minimally invasive implantation, programmable stimulation, and high success rate in managing neuropathic pain. The segment also benefits from continuous technological advancements such as wireless programming, MRI compatibility, and closed-loop feedback systems, which enhance patient outcomes and clinician confidence. Moreover, the large target patient pool, including post-surgical pain and peripheral neuropathy cases, contributes to the high demand.
The deep brain stimulator segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by increasing prevalence of movement disorders such as Parkinson’s disease and dystonia in Japan, China, and South Korea. These devices provide precise neuromodulation for motor symptom management, improving patient quality of life. The growing number of neurology specialty centers and rising awareness of deep brain stimulation therapy are further accelerating adoption. Technological innovations including smaller implantable systems and enhanced battery life make these devices more attractive. The rising geriatric population and government support programs for neurological care in Asia-Pacific are also significant growth enablers.
- By Lead Type
On the basis of lead type, the market is segmented into percutaneous and paddle leads. The percutaneous lead segment dominated the market in 2025, accounting for the majority of installations due to its minimally invasive nature and shorter recovery time. Percutaneous leads are widely preferred for spinal cord stimulators because they can be implanted through small incisions, reducing surgical complications and hospital stay. Hospitals and clinics favor these leads for their flexibility in placement and ease of removal or replacement when needed. Their compatibility with both temporary and permanent stimulation systems further supports high demand. For instance, many chronic pain clinics in China and India prefer percutaneous leads for outpatient procedures.
The paddle lead segment is expected to witness the fastest growth during 2026–2033, driven by its superior stability and precise stimulation coverage, particularly in complex spinal cord pain cases. Paddle leads are commonly used in advanced neuromodulation therapies requiring targeted electrode placement. Technological improvements in minimally invasive paddle implantation techniques are reducing past surgical challenges. Rising awareness among neurosurgeons and the increasing number of specialized spine centers in Japan and South Korea contribute to accelerated adoption.
- By Biomaterial
On the basis of biomaterial, the market is segmented into metallic, polymeric, and ceramic devices. The metallic segment dominated the market in 2025 with the largest share due to its established biocompatibility, durability, and ability to conduct electrical stimulation effectively over long periods. Metallic electrodes and implants are widely used in spinal cord and deep brain stimulators for reliable therapy outcomes. Hospitals and clinics prefer metallic devices for long-term implants as they ensure minimal degradation and consistent stimulation. The segment also benefits from ongoing innovations in coating and alloy materials to reduce tissue inflammation and improve device longevity.
The polymeric segment is anticipated to witness the fastest growth from 2026 to 2033, driven by the development of flexible, lightweight, and MRI-compatible polymer-based leads and implants. Polymers allow better patient comfort and reduce the risk of tissue damage. The rising focus on wearable and patient-centric devices, particularly in home-based neuromodulation therapy, is contributing to growth. Emerging polymeric devices in Japan and China are increasingly preferred in clinical trials for both spinal cord and vagus nerve stimulation.
- By Application
On the basis of application, the market is segmented into failed back surgery syndrome, Parkinson’s disease, urinary incontinence, epilepsy, and gastroparesis. The failed back surgery syndrome segment dominated the market in 2025 due to the high prevalence of chronic post-surgical pain in China, India, and Japan. Spinal cord stimulators are particularly effective for this condition, providing targeted pain relief where conventional therapies fail. Hospitals prioritize these devices for their proven clinical outcomes, minimally invasive implantation, and ability to reduce reliance on long-term opioid therapy. For instance, many pain management centers in South Korea utilize spinal cord stimulation as a standard of care for post-lumbar surgery patients. The segment also benefits from technological advancements such as closed-loop stimulation systems for adaptive pain management.
The Parkinson’s disease segment is expected to witness the fastest growth from 2026 to 2033, fueled by rising prevalence of movement disorders in the ageing population of Japan, China, and South Korea. Deep brain stimulators provide effective symptom management, improving motor control and quality of life. Increasing government programs for neurological disorders, clinical research investments, and awareness campaigns in hospitals and neurology clinics are accelerating adoption. Technological innovations such as smaller implantable systems and rechargeable batteries make therapy more patient-friendly, supporting growth in this segment.
- By End-User
On the basis of end-user, the market is segmented into hospitals, clinics, home healthcare, and community healthcare centers. The hospital segment dominated the market in 2025 with the largest revenue share, owing to the availability of advanced surgical infrastructure, skilled neurosurgeons, and high patient throughput for neuromodulation procedures. Hospitals in Japan, China, and South Korea often serve as primary adoption centers for spinal cord and deep brain stimulators due to clinical trial collaborations and post-operative care facilities. The segment benefits from strong partnerships with device manufacturers for training and long-term patient management.
The home healthcare segment is expected to witness the fastest growth from 2026 to 2033, driven by the rising demand for remote monitoring and at-home management of chronic pain and neurological disorders. Wearable neuromodulation systems and wireless programmable devices enable patients to manage therapy outside hospital settings, reducing the need for frequent visits. Growth is supported by telemedicine adoption, mobile app integration, and increasing awareness of patient-centric care models in India, China, and Australia. Technological advancements and reduced device sizes are making home-based therapy increasingly feasible and convenient.
Asia-Pacific Internal Neuromodulation Devices Market Regional Analysis
- Japan dominated the Asia-Pacific internal neuromodulation devices market with the largest revenue share of 30.2% in 2025, characterized by advanced healthcare infrastructure, strong reimbursement policies, and early adoption of innovative neuromodulation therapies, with hospitals and specialty clinics increasingly implementing these devices for improved patient outcomes
- Patients and clinicians in the region highly value the clinical efficacy, minimally invasive implantation, and programmable features offered by internal neuromodulation devices, which improve treatment outcomes for conditions such as failed back surgery syndrome, Parkinson’s disease, and epilepsy
- This widespread adoption is further supported by growing awareness of advanced therapies, increasing healthcare expenditure, and government initiatives to improve neurological care, establishing internal neuromodulation devices as a preferred solution for hospitals, specialty clinics, and pain management centers across China, Japan, India, and South Korea
The China Internal Neuromodulation Devices Market Insight
The China internal neuromodulation devices market captured the largest revenue share of 30.2% in 2025, fueled by the rising prevalence of chronic pain, neurological disorders, and increasing hospital infrastructure. Patients and clinicians are prioritizing advanced implantable therapies, including spinal cord stimulators and deep brain stimulators, to improve clinical outcomes. The growing adoption of minimally invasive procedures and remote programmable devices, combined with expanding specialty neurology and pain management centers, further drives the market. Additionally, government initiatives promoting neurological healthcare and digital health integration are enhancing accessibility. With continuous technological advancements and local manufacturing, internal neuromodulation devices are becoming increasingly affordable and widely adopted across China.
Japan Internal Neuromodulation Devices Market Insight
The Japan internal neuromodulation devices market is gaining momentum due to the country’s advanced healthcare infrastructure, rapid urbanization, and high patient awareness of chronic neurological disorders. Hospitals and clinics emphasize precision therapies, particularly for Parkinson’s disease and movement disorders, driving demand for deep brain stimulators. Integration of programmable and MRI-compatible devices into hospital workflows enhances treatment outcomes and reduces clinician workload. Japan’s ageing population and rising geriatric care requirements are expected to further spur adoption in both residential and clinical settings. Moreover, collaborations between device manufacturers and healthcare providers are promoting clinical research and training programs, accelerating market growth.
India Internal Neuromodulation Devices Market Insight
The India internal neuromodulation devices market accounted for the largest market share in South Asia in 2025, attributed to rising healthcare expenditure, increasing awareness of chronic pain management, and rapid expansion of hospitals and specialty clinics. Internal neuromodulation devices are being increasingly adopted for failed back surgery syndrome, epilepsy, and urinary incontinence. The government’s push for digital health initiatives, coupled with growing investments in advanced medical technologies, is boosting accessibility. Affordable device options and the presence of domestic and international manufacturers are further encouraging adoption. Additionally, the increasing geriatric population and rising incidence of neurological disorders are key factors supporting sustained growth.
South Korea Internal Neuromodulation Devices Market Insight
The South Korea internal neuromodulation devices market is expected to grow at a considerable CAGR during the forecast period, driven by high awareness of advanced therapies, well-developed healthcare infrastructure, and increasing investment in neurological research. Hospitals and pain management centers are rapidly adopting spinal cord stimulators and vagus nerve stimulators to treat chronic pain and epilepsy. The integration of programmable and wireless-enabled devices facilitates improved patient care and post-operative monitoring. Rising demand for minimally invasive therapies and government initiatives supporting neurological care further propel market growth. Strategic collaborations between manufacturers and hospitals for clinical trials are also enhancing adoption.
Asia-Pacific Internal Neuromodulation Devices Market Share
The Asia-Pacific Internal Neuromodulation Devices industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Boston Scientific Corporation (U.S.)
- Abbott (U.S.)
- Nevro Corp. (U.S.)
- LivaNova, plc (U.K.)
- NeuroPace, Inc. (U.S.)
- NeuroSigma, Inc. (U.S.)
- Synapse Biomedical Inc. (U.S.)
- Axonics, Inc. (U.S.)
- Saluda Medical Pty Limited (Australia)
- Mainstay Medical International plc (Ireland)
- Aleva Neurotherapeutics SA (Switzerland)
- Nuvectra Corporation (U.S.)
- Bioness Inc. (U.S.)
- Soterix Medical Inc. (U.S.)
- SPR Therapeutics (U.S.)
- Biotronik SE & Co. KG (Germany)
- NeuroMetrix, Inc. (U.S.)
- EndoStim Inc. (U.S.)
- ElectroCore Inc. (U.S.)
What are the Recent Developments in Asia-Pacific Internal Neuromodulation Devices Market?
- In October 2025, China’s National Medical Products Administration (NMPA) approved the Nerivio® REN wearable for acute migraine treatment, marking the first regulatory clearance of Remote Electrical Neuromodulation wearable technology in China and offering a drug‑free, smartphone‑controlled migraine solution for millions of patients
- In January 2024, the Japanese Ministry of Health, Labour and Welfare approved Neurolief’s Relivion® neuromodulation device for at‑home use in the acute treatment of migraines, making it the first such home‑use neuromodulation device approved in Japan and integrating mobile monitoring and AI capabilities
- In August 2023, the 16th Annual Scientific Meeting of the Neuromodulation Society of Australia and New Zealand was held, bringing together clinicians and researchers to share clinical data and technological advances in neuromodulation therapies across the Asia‑Pacific region
- In June 2023, the Neuromodulation Society of Australia and New Zealand (NSANZ) announced plans to develop a centralized neuromodulation device registry, aimed at collecting long‑term clinical outcomes for neuromodulation therapies and positioning Australia as a leader in real‑world evidence generation outside Europe
- In December 2022, Sawai Pharmaceutical applied for marketing and manufacturing approval of a non‑invasive neuromodulation device (SWD001) with Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), progressing regulatory efforts for novel neuromodulation technologies in Japan prior to the subsequent 2024 approval of Relivion
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.