Asia Pacific Multiplex Assays Market
Market Size in USD Million
CAGR :
% 
 USD
750.50 Million
USD
1,562.55 Million
2024
2032
USD
750.50 Million
USD
1,562.55 Million
2024
2032
| 2025 –2032 | |
| USD 750.50 Million | |
| USD 1,562.55 Million | |
|
|
|
|
Asia-Pacific Multiplex Assays Market Size
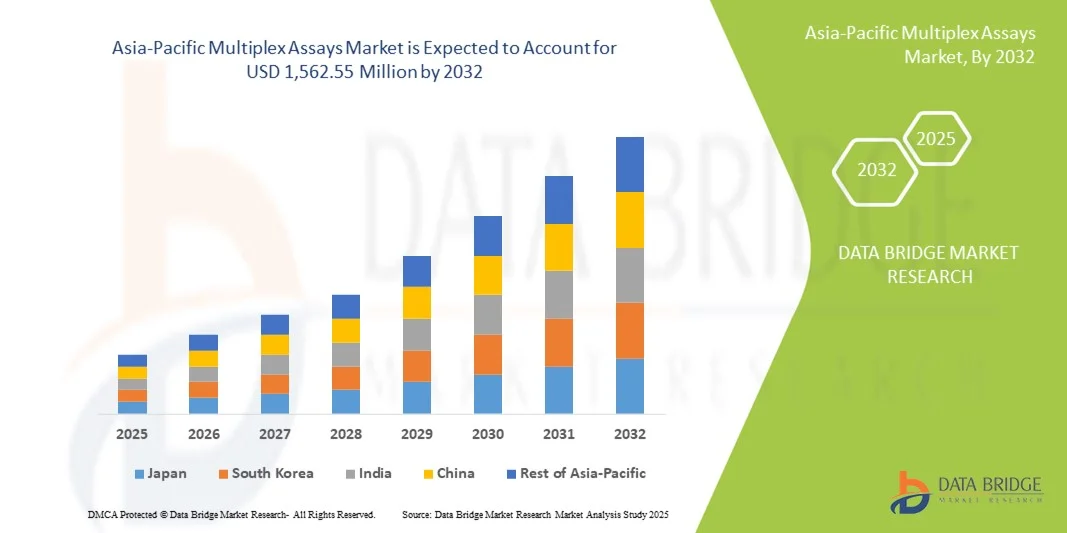
- The Asia-Pacific multiplex assays market size was valued at USD 750.50 million in 2024 and is expected to reach USD 1,562.55 million by 2032, at a CAGR of 9.6% during the forecast period
- The market growth is largely fueled by the increasing prevalence of chronic and infectious diseases, rising demand for personalized medicine, and technological advancements in diagnostic platforms, driving adoption across clinical and research applications
- Furthermore, expanding healthcare infrastructure, government initiatives for early disease detection, and the rising need for efficient, high-throughput diagnostic solutions are positioning multiplex assays as a preferred tool in both clinical diagnostics and research settings, thereby significantly boosting the industry's growth
Asia-Pacific Multiplex Assays Market Analysis
- Multiplex assays, enabling the simultaneous detection of multiple analytes in a single test, are becoming essential tools in modern diagnostics and biomedical research due to their high-throughput capabilities, accuracy, and efficiency in both clinical and research settings
- The growing demand for multiplex assays is primarily driven by the increasing prevalence of chronic and infectious diseases, rising need for personalized medicine, and continuous technological advancements in assay platforms and detection methods
- China dominated the Asia-Pacific multiplex assays market with the largest revenue share of 39% in 2024, supported by robust healthcare infrastructure, government initiatives for early disease detection, and rapid adoption of advanced diagnostic technologies, with clinical diagnostics and research laboratories witnessing significant growth in assay implementation
- India is expected to be the fastest-growing region in the Asia-Pacific multiplex assays market during the forecast period due to increasing healthcare expenditure, rising awareness about early diagnostics, and expanding laboratory networks
- Reagents and Consumables segment dominated the multiplex assays market with a market share of 51.7% in 2024, driven by recurring demand for reagents, kits, and assay components in both routine diagnostics and research applications
Report Scope and Asia-Pacific Multiplex Assays Market Segmentation
|
Attributes |
Asia-Pacific Multiplex Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Multiplex Assays Market Trends
Integration with High-Throughput and AI-Driven Platforms
- A significant and accelerating trend in the Asia-Pacific multiplex assays market is the integration of assays with high-throughput automated systems and AI-driven data analysis platforms. This combination enhances efficiency, accuracy, and speed in clinical diagnostics and biomedical research
- For instance, Luminex xMAP technology coupled with AI-enabled analytics allows simultaneous detection of multiple biomarkers and rapid interpretation of complex datasets. Similarly, Quanterix Simoa platforms utilize AI to improve signal detection sensitivity, enabling precise quantification of low-abundance analytes
- AI integration in multiplex assays facilitates predictive insights, pattern recognition in disease biomarkers, and streamlined workflow management. For instance, some Bio-Rad multiplex platforms can flag abnormal results and optimize assay parameters over time, improving diagnostic reliability
- The seamless integration of multiplex assays with laboratory information management systems (LIMS) and broader digital diagnostic platforms allows centralized monitoring and data management, enhancing laboratory workflow efficiency and enabling high-throughput analysis
- This trend towards more automated, intelligent, and interconnected assay systems is redefining expectations for clinical and research diagnostics. Companies such as Thermo Fisher Scientific are developing multiplex assay solutions with AI-enabled data interpretation and high-throughput automation for faster and more reliable results
- The demand for multiplex assays integrated with AI and automated platforms is growing rapidly across clinical diagnostics, pharmaceutical research, and academic laboratories, as stakeholders increasingly prioritize efficiency, accuracy, and comprehensive analytical capabilities
Asia-Pacific Multiplex Assays Market Dynamics
Driver
Rising Need for Efficient Disease Diagnostics and Personalized Medicine
- The increasing prevalence of chronic and infectious diseases, coupled with the growing emphasis on personalized medicine, is a major driver for the heightened demand for multiplex assays
- For instance, in March 2024, Qiagen launched a multiplex panel for respiratory infections optimized for high-throughput diagnostic laboratories in Asia-Pacific, highlighting the adoption of advanced multiplex solutions to address regional healthcare needs
- As healthcare providers aim for faster, more accurate diagnosis and treatment planning, multiplex assays offer simultaneous detection of multiple biomarkers, reducing turnaround time and improving clinical decision-making
- Furthermore, the adoption of multiplex assays in clinical research, drug development, and vaccine evaluation is accelerating, driven by the need for comprehensive biomarker profiling and cost-efficient testing solutions
- The versatility of multiplex assays for both research and clinical applications, along with their compatibility with automated systems, is propelling their adoption across hospitals, diagnostic labs, and research institutes in the region
Restraint/Challenge
High Cost of Advanced Assays and Regulatory Compliance Hurdles
- The relatively high cost of advanced multiplex assay kits and instrumentation, compared to conventional singleplex tests, poses a significant barrier to adoption, especially in price-sensitive markets within Asia-Pacific
- For instance, sophisticated platforms from Luminex or Quanterix can be cost-prohibitive for smaller laboratories or emerging markets
- In addition, stringent regulatory requirements for diagnostic assays, including approvals from regional authorities such as China NMPA, India CDSCO, and ASEAN regulatory bodies, can delay market entry and commercialization
- Addressing cost barriers through affordable kits, scalable platforms, and partnerships with local distributors, alongside compliance with regulatory standards, is essential for wider adoption and market growth
- While technological advancements continue to enhance assay performance, overcoming pricing and regulatory challenges will be critical to achieving broader penetration in the Asia-Pacific multiplex assays market
- Limited technical expertise in emerging markets is another challenge, as effective use of multiplex assays requires trained personnel and proper laboratory infrastructure
- Fragmented healthcare systems and variable reimbursement policies across countries in the region can restrict large-scale adoption despite technological advancements
Asia-Pacific Multiplex Assays Market Scope
The market is segmented on the basis of products and services, type, technology, application, and end user.
- By Products and Services
On the basis of products and services, the Asia-Pacific multiplex assays market is segmented into reagents and consumables, instruments and accessories, and software and services. The reagents and consumables segment dominated the market with the largest revenue share of 51.7% in 2024, driven by the recurring demand for assay kits, antibodies, primers, and detection reagents in both clinical diagnostics and research laboratories. Consumables are essential for the daily operation of multiplex assays, and their frequent replacement ensures steady revenue for manufacturers. The versatility of reagents and consumables allows them to support multiple assay types and technologies, increasing their utility across different applications. Growing prevalence of chronic and infectious diseases in Asia-Pacific has increased reliance on consumables for routine diagnostic and high-throughput testing. In addition, consumables are often bundled with instruments and platforms, creating recurring revenue streams for manufacturers.
The software and services segment is expected to witness the fastest growth during the forecast period, fueled by the increasing integration of AI and high-throughput data analysis platforms. Software solutions enable laboratories to process complex multiplex assay datasets efficiently, reduce errors, and provide actionable insights. Managed services, training, and technical support are also gaining traction, particularly in emerging markets where laboratories require external expertise to implement multiplex platforms. The shift toward digital diagnostics and automated workflows further drives the adoption of software and services. Cloud-based software solutions also facilitate remote monitoring and centralized management of multiplex data. These factors collectively contribute to the rapid growth of the software and services segment.
- By Type
On the basis of type, the market is segmented into nucleic acid multiplex assays, protein multiplex assays, and cell-based multiplex assays. The nucleic acid multiplex assays segment dominated the market in 2024 with the largest revenue share of 44%, primarily due to their widespread use in detecting infectious diseases, genetic disorders, and pathogen profiling. Nucleic acid assays offer high sensitivity and specificity, making them indispensable in clinical diagnostics and public health laboratories. Their ability to deliver rapid and simultaneous detection of multiple targets reduces sample volume requirements and turnaround time. The COVID-19 pandemic further accelerated adoption, emphasizing the need for multiplex pathogen detection. In addition, government screening programs in the region are increasingly relying on nucleic acid multiplex platforms. The segment’s reliability and established protocols make it a preferred choice for hospitals and diagnostic labs.
The protein multiplex assays segment is anticipated to witness the fastest CAGR from 2025 to 2032, driven by the growing demand for biomarker discovery, personalized medicine, and therapeutic monitoring. Protein assays are critical for studying disease progression, drug response, and immune profiling in research and clinical settings. Technological advancements such as microarrays and multiplex immunoassays have enhanced their throughput, accuracy, and reproducibility. Increasing investment in R&D, especially in oncology and immunology research, fuels the demand for protein assays. Rising awareness about chronic disease management in Asia-Pacific also contributes to adoption. Collaborations between assay providers and research institutes to develop region-specific protein panels further boost market growth.
- By Technology
On the basis of technology, the market is segmented into protein microarray, polymerase chain reaction (PCR), multiplex real-time PCR, flow cytometry, fluorescence detection, luminescence, and others. The multiplex real-time PCR segment dominated the market with the largest share of 42.5% in 2024, owing to its high sensitivity, rapid turnaround time, and ability to detect multiple nucleic acid targets simultaneously. Multiplex real-time PCR is widely applied in infectious disease diagnostics, genetic testing, and pathogen surveillance. Its automation compatibility and scalability make it ideal for hospitals and clinical laboratories. The platform reduces manual errors and improves workflow efficiency. Continuous technological innovations, such as improved probes and chemistries, further enhance its accuracy. Government-supported infectious disease testing programs have also driven adoption of this technology.
The flow cytometry segment is expected to witness the fastest growth during the forecast period, driven by its increasing use in cell-based assays, immunophenotyping, and high-content analysis. Flow cytometry enables simultaneous measurement of multiple parameters at the single-cell level, offering detailed insights into cellular function and disease mechanisms. It is widely adopted in immuno-oncology, vaccine development, and stem cell research. The demand is also fueled by automated high-throughput flow cytometry systems that reduce processing time. The integration of AI for data analysis further improves its appeal in research and clinical applications. Rising collaborations between manufacturers and research institutes in Asia-Pacific are accelerating adoption.
- By Application
On the basis of application, the market is segmented into clinical diagnostics and research and development. The clinical diagnostics segment dominated the market in 2024 with the largest share of 55%, as hospitals and clinical laboratories increasingly rely on multiplex assays for rapid, accurate, and cost-effective detection of infectious diseases, genetic disorders, and chronic disease biomarkers. Multiplex assays allow simultaneous testing of multiple analytes, reducing sample volumes and turnaround time. Government initiatives for early disease detection and national screening programs further boost adoption. Increasing patient awareness regarding timely diagnostics is another key factor. Compatibility with automated platforms enhances efficiency and reduces operational costs. The segment’s dominance is reinforced by partnerships between multiplex assay providers and large hospital chains.
The research and development segment is expected to witness the fastest growth from 2025 to 2032, driven by the expanding focus on biomarker discovery, drug development, and personalized medicine. Pharmaceutical and biotechnology companies, along with academic research institutes, are increasingly investing in multiplex assays to accelerate translational research. Integration with high-throughput platforms and AI-driven data analytics reduces experimental timelines and enhances accuracy. Rising government and private funding for biomedical research in Asia-Pacific supports market growth. Demand for multiplex platforms in vaccine development and immune profiling is also increasing. Collaborations between assay developers and research organizations are strengthening the segment’s growth trajectory.
- By End User
On the basis of end user, the market is segmented into hospitals, clinical laboratories, pharmaceutical and biotechnology companies, research institutes, and others. The hospitals segment dominated the market in 2024 with the largest revenue share of 48%, driven by the increasing demand for rapid, multiplexed diagnostic testing in patient care. Hospitals benefit from multiplex assays as they reduce turnaround time, allow simultaneous testing of multiple conditions, and improve patient management outcomes. Growing numbers of private and public hospitals in Asia-Pacific, coupled with rising patient awareness, further support this segment. Compatibility with automated and high-throughput platforms enhances operational efficiency. National healthcare programs emphasizing early disease detection further boost adoption. Hospitals also favor multiplex assays for their ability to reduce sample volume and testing costs.
The pharmaceutical and biotechnology companies segment is expected to witness the fastest growth during the forecast period, fueled by the rising use of multiplex assays in drug discovery, clinical trials, and therapeutic monitoring. Companies leverage multiplex technologies for high-throughput biomarker screening, immune profiling, and vaccine development. Increasing R&D investments and collaborations with multiplex assay providers further accelerate adoption. Advanced protein and nucleic acid assays are particularly attractive for precision medicine initiatives. The growing focus on translational research and personalized therapeutics in Asia-Pacific also supports expansion. Multiplex platforms offer scalability and reproducibility, which are critical for pharmaceutical applications.
Asia-Pacific Multiplex Assays Market Regional Analysis
- China dominated the Asia-Pacific multiplex assays market with the largest revenue share of 39% in 2024, supported by robust healthcare infrastructure, government initiatives for early disease detection, and rapid adoption of advanced diagnostic technologies, with clinical diagnostics and research laboratories witnessing significant growth in assay implementation
- Countries such as China, India, and Japan are witnessing rapid expansion of hospital networks, clinical laboratories, and research institutes, which are increasingly implementing multiplex assays for disease diagnostics, biomarker analysis, and therapeutic monitoring
- The widespread adoption is further supported by government initiatives for early disease detection, national screening programs, and investments in personalized medicine, making multiplex assays a preferred solution in both clinical and research settings
Japan Multiplex Assays Market Insight
The Japan multiplex assays market is gaining momentum due to the country’s technologically advanced healthcare infrastructure, high awareness of diagnostic innovation, and demand for accurate and rapid disease detection. The adoption is driven by an increasing number of hospitals and research centers implementing multiplex platforms for biomarker profiling and personalized medicine. Integration with AI and automated laboratory systems is facilitating workflow efficiency, while government policies supporting healthcare innovation further stimulate market growth.
India Multiplex Assays Market Insight
The India multiplex assays market accounted for the largest market revenue share in the Asia-Pacific region in 2024, supported by expanding hospital networks, growing clinical laboratory services, and increasing government initiatives for disease screening and healthcare modernization. The rise of private diagnostic chains and research institutes adopting multiplex technologies is driving demand. Affordable assay kits and regional manufacturing of components are improving accessibility, while the country’s focus on high-throughput diagnostics and infectious disease monitoring supports rapid growth.
South Korea Multiplex Assays Market Insight
The South Korea multiplex assays market is expanding due to the country’s advanced healthcare system, rapid adoption of high-throughput diagnostics, and strong government initiatives for early disease detection and personalized medicine. Hospitals and research institutes are leveraging multiplex assays for clinical diagnostics, drug development, and vaccine research. Integration with digital laboratory management systems and AI-based analytics further enhances adoption. Strong collaboration between domestic manufacturers and research organizations is strengthening the market presence.
Asia-Pacific Multiplex Assays Market Share
The Asia-Pacific Multiplex Assays industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- QIAGEN (Germany)
- Abcam Limited. (U.K.)
- BD (U.S.)
- PerkinElmer (U.S.)
- Promega Corporation (U.S.)
- Bio-Techne Corporation (U.S.)
- Agilent Technologies, Inc. (U.S.)
- Sysmex Corporation (Japan)
- Olink Holding AB (Sweden)
- Randox Laboratories Ltd (U.K.)
- Seegene Inc. (South Korea)
- Meso Scale Diagnostics, LLC (U.S.)
- Advanced Cell Diagnostics, Inc. (U.S.)
- R&D Systems, Inc. (U.S.)
- Assay Genie (Ireland)
- Ayoxxa Biosystems GmbH (Germany)
- Luminex Corporation (U.S.)
What are the Recent Developments in Asia-Pacific Multiplex Assays Market?
- In September 2025, The World Health Organization (WHO) announced the development of guidelines on multiplex testing. The guidelines aim to provide recommendations and guidance on multiplex testing by providers and self-testers, create key principles, and address issues of integration while achieving public health impact. This initiative underscores the global emphasis on enhancing diagnostic capabilities, particularly in the Asia-Pacific region, to address the growing burden of infectious diseases
- In September 2025, QIAGEN and the Asia Pacific Association of Gastroenterology (APAGE) convened a consensus meeting with leading experts to discuss advancements in rapid molecular syndromic testing for gastrointestinal diseases. This collaboration aims to enhance diagnostic capabilities in the region
- In April 2024, Bio-Rad Laboratories launched the ddPLEX ESR1 Mutation Detection Kit, an ultrasensitive multiplexed digital PCR assay designed for detecting breast cancer mutations in clinical research. This kit enables simultaneous detection, discrimination, and absolute quantification of seven known relevant ESR1 mutations, with an analytical sensitivity down to 0.01% variant allele fraction (VAF). It allows for same-day results from circulating tumor DNA (ctDNA) in plasma or DNA from formalin-fixed paraffin-embedded (FFPE) tissue samples
- In March 2023, Luminex Corporation introduced the xMAP INTELLIFLEX DR-SE system, an advanced platform designed to enhance the flexibility and accuracy of multiplex assays. This system offers improved sensitivity and throughput, supporting the growing demand for comprehensive testing methods in diagnostics and research sectors
- In May 2022, QuantuMDx introduced the Q-POC™ SARS-CoV-2, Flu A/B & RSV Assay, a point-of-care multiplex PCR test capable of delivering accurate results in approximately 35 minutes. This assay detects SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus (RSV) simultaneously, offering a simple workflow suitable for use by healthcare professionals after basic training
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.