Asia Pacific Pcr Multiplex Assays Market
Market Size in USD Million
CAGR :
% 
 USD
1.21 Million
USD
2.42 Million
2024
2032
USD
1.21 Million
USD
2.42 Million
2024
2032
| 2025 –2032 | |
| USD 1.21 Million | |
| USD 2.42 Million | |
|
|
|
|
Asia-Pacific Polymerase Chain Reaction (PCR) Multiplex Assays Market Analysis
A multiplex assay can amplify more than one target sequence by using numerous primer pairs in reaction mixes. The PCR technique saves time and effort in laboratory investigations, making it a useful and quick screening tool for clinical and research labs. Manufacturers of PCR multiplex assays are looking to expand their business and provide new services.
Polymerase Chain Reaction (PCR) Multiplex Assays Market Size
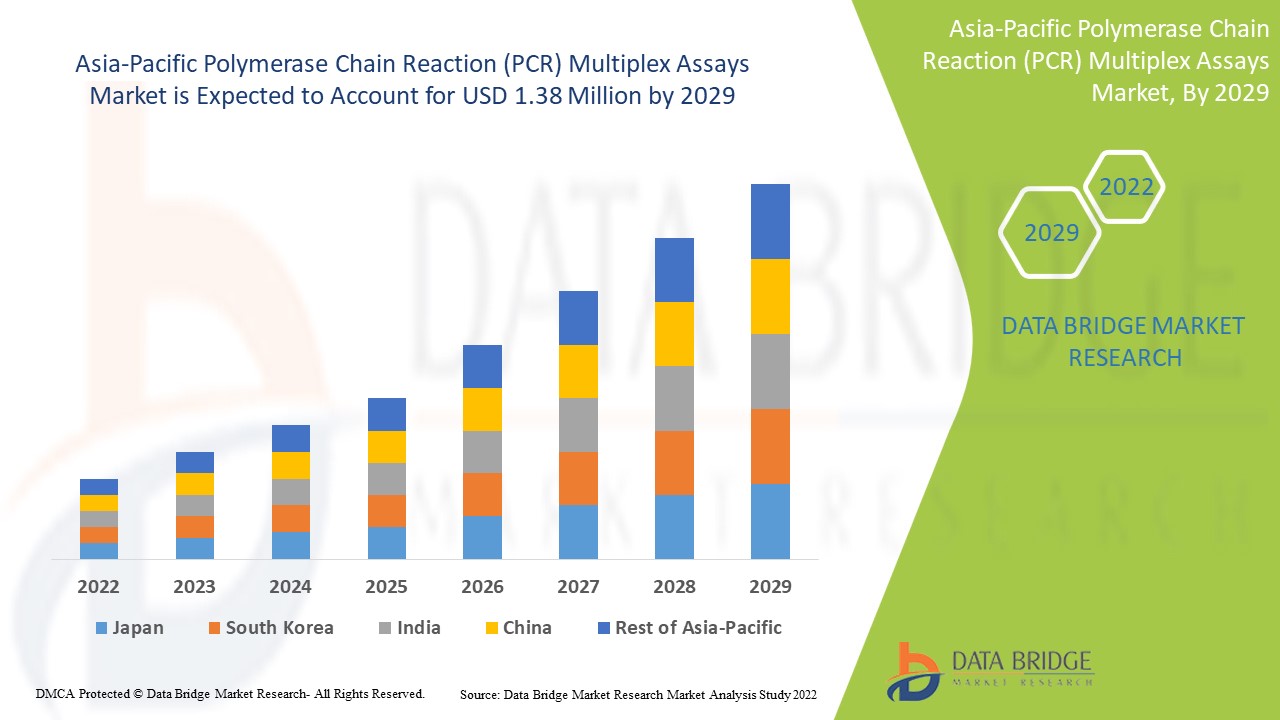
Asia-Pacific Polymerase Chain Reaction (PCR) Multiplex Assays market size was valued at USD 1.21 million in 2024 and is projected to reach USD 2.42 million by 2032, with a CAGR of 9.1% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Polymerase Chain Reaction (PCR) Multiplex Assays Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel |
|
Key Market Players |
Hologic, Inc. (U.S), Luminex (U.S), BD (U.S), Abbott (U.S), Thermo Fisher Scientific Inc. (U.S), QIAGEN (Germany), Agilent Technologies Inc. (U.S), MESO SCALE DIAGNOSTICS, LLC (U.S), Randox Laboratories Ltd. (U.K), Seegene Inc. (Korea), Illumina, Inc (U.S), Bio-Rad Laboratories, Inc. (U.S), Quest Diagnostics Incorporated (U.S), Abcam Plc (U.K) |
|
Market Opportunities |
|
Polymerase Chain Reaction (PCR) Multiplex Assays Market Definition
Multiplex assays are a new technology that allows you to run multiple target sequences in a single step. The evolution of PCR multiplex assays in terms of smart features such as voice command, facial authentication, and radio-frequency identification (RFID) features has shifted the system from a manual instrument to an automated PCR multiplexes system.
Polymerase Chain Reaction (PCR) Multiplex Assays Market Dynamics
Drivers
- Growing number of hospitals across emerging economies
Healthcare technological breakthroughs, a growing research and development industry, expanding healthcare reforms, and a rising need for healthcare infrastructure will likely propel the regional market forward.
- Growing incidences of chronic illness
Multiplex assays in companion diagnostics are becoming more popular due to the rising prevalence of cancer. Additionally, the advantages of multiplex assays over singleplex and traditional assays, technological advancements, rising healthcare expenditure, and rising awareness about the multifactorial nature of various diseases and pathological conditions are expected to drive the Multiplex Assays market forward.
Opportunities
Expanded government financing, increasing pharmaceutical and biotechnology research activities, and a greater focus on improving experiment efficiency will propel the business forward. Because of the growing recognition of the multifactorial character of illnesses and pathological situations like cancer and Alzheimer's disease, a cost-effective and time-saving technique for evaluating many analyzers in a single sample is needed.
Restraints/Challenges
High equipment prices and a scarcity of experienced workers could stymie the expansion of the multiplex assays business in the near future.
This Polymerase Chain Reaction (PCR) multiplex assays market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the PCR multiplex assays market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Asia-Pacific Polymerase Chain Reaction (PCR) Multiplex Assays Market Scope
The Polymerase Chain Reaction (PCR) multiplex assays market is segmented on the basis of type, product, application and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Reagents and Consumables
- Instruments and Accessories
- Software and Services
Type
- Protein Based Multiplex Assays
- Nucleic Acid-Based Multiplex Assay
- Others
End-User
- Pharmaceutical and Biotechnology Companies
- Research Institutes
- Clinical Laboratories
- Hospitals
Application
- Research and Development
- Clinical Diagnostics
- Companion Diagnostics
Polymerase Chain Reaction (PCR) Multiplex Assays Market Regional Analysis
The Polymerase Chain Reaction (PCR) multiplex assays market is analyzed and market size insights and trends are provided by country, type, product, application and end user as referenced above.
The countries covered in the Polymerase Chain Reaction (PCR) multiplex assays market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
The rising usage of multiplex assay, North America is likely to maintain its market share dominance over the forecast period. Other reasons, such as the improvement of technology, the presence of well-developed healthcare infrastructures, and the increased prevalence of various chronic diseases, are expected to boost the multiplex assay market in this area.
Due to the increased usage of companion diagnostics, Asia Pacific is predicted to develop at a rapid pace, with an increasing market share throughout the forecast period. Furthermore, the multiplex assay market in this area is predicted to rise due to rising acceptance of advanced technologies, skyrocketing need for safe and effective treatment choices, and new product launches.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Polymerase Chain Reaction (PCR) Multiplex Assays Market Share
The Polymerase Chain Reaction (PCR) multiplex assays market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Polymerase Chain Reaction (PCR) multiplex assays market.
Polymerase Chain Reaction (PCR) Multiplex Assays Market Leaders Operating in the Market Are:
- Hologic, Inc. (U.S)
- Luminex (U.S)
- BD (U.S)
- Abbott (U.S)
- Thermo Fisher Scientific Inc. (U.S)
- QIAGEN (Germany)
- Agilent Technologies Inc. (U.S)
- MESO SCALE DIAGNOSTICS, LLC (U.S)
- Randox Laboratories Ltd. (U.K)
- Seegene Inc. (Korea)
- Illumina, Inc (U.S)
- Bio-Rad Laboratories, Inc. (U.S)
- Quest Diagnostics Incorporated (U.S)
- Abcam Plc (U.K)
Latest Developments in Polymerase Chain Reaction (PCR) Multiplex Assays Market
- Olink announced in December 2018 that oncology's 92-plex protein biomarker panel is now accessible as kits or as an analysis service. In two specialized panels, this novel biomarker panel can test 184 proteins. This novel assays panel covers a wide range of oncology-related diseases and biological processes.
- PerkinElmer, Inc. announced in October 2014 that they would be exhibiting Omics Office software in London; it is new and revolutionary software for qPCR and digital PCR Congress. This new programme assists scientists in importing, analysing, and validating complex data in order to make informed conclusions. This software can help you visualise and analyse biological data at a high level of sophistication. The company's product portfolio has been expanded in the market as a result of the debut of this product.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.