Europe Anesthesia And Respiratory Devices Market
Market Size in USD Million
CAGR :
% 
 USD
0.99 Million
USD
2.00 Million
2024
2032
USD
0.99 Million
USD
2.00 Million
2024
2032
| 2025 –2032 | |
| USD 0.99 Million | |
| USD 2.00 Million | |
|
|
|
|
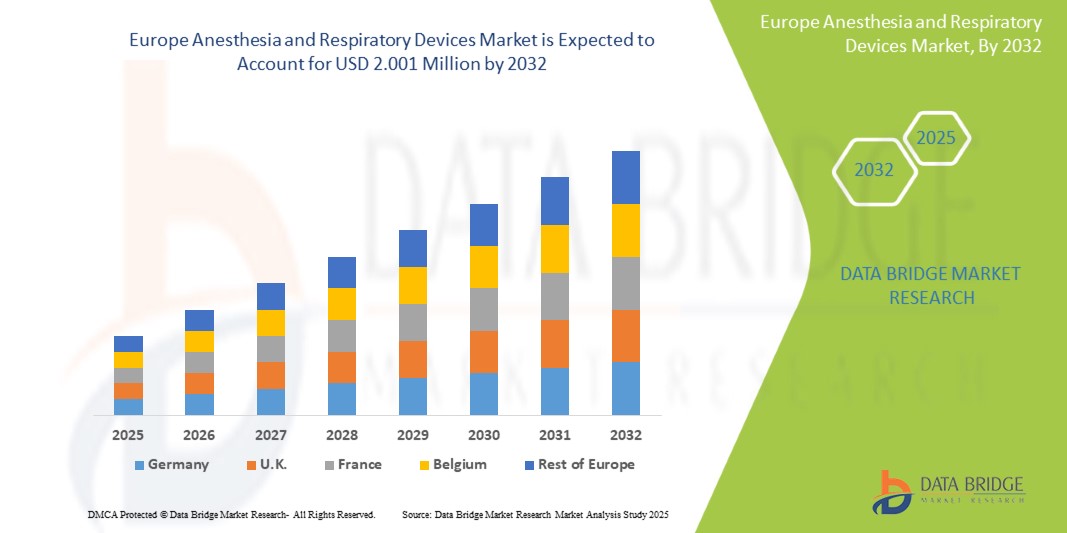
Anesthesia and Respiratory Devices Market Size
- The Europe Anesthesia and Respiratory Devices Market was valued at USD 0.986 Million in 2024 and is expected to reach USD 2.001 Million by 2032.
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 6.35%, primarily driven by the increasing prevalence of respiratory disorders, aging population, and rising surgical procedures across the region
- Growth is further supported by technological advancements in anesthesia and ventilation systems, rising healthcare expenditure, and the increased adoption of portable and home-care respiratory devices amid a growing trend toward personalized care
Anesthesia and Respiratory Devices Market Analysis
- Anesthesia and respiratory devices are critical components in perioperative care and chronic disease management, extensively used in hospitals, ambulatory surgical centers, and home care settings
- The product portfolio includes anesthesia machines, ventilators, nebulizers, CPAP/BiPAP machines, oxygen concentrators, and respiratory disposables, all essential for delivering precise and controlled respiratory support during surgery or chronic respiratory therapy
- Demand for these devices is fueled by rising incidences of chronic respiratory diseases such as COPD and sleep apnea, post-pandemic emphasis on respiratory health, and a shift toward non-invasive ventilation and home-based respiratory therapy solutions
- Additionally, Europe’s strong focus on medical device innovation, supportive regulatory frameworks, and expanding elderly population base are further contributing to market expansion
Report Scope and Anesthesia and Respiratory Devices Market Segmentation
|
Attributes |
Anesthesia and Respiratory Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Anesthesia and Respiratory Devices Market Trends
“Rising Demand for Portable and Home-Based Respiratory Care Solutions”
- There is a growing shift toward portable, compact, and user-friendly respiratory and anesthesia devices, driven by an aging population, rising cases of chronic respiratory conditions, and increasing demand for home healthcare
- Technological advancements are enabling the development of lightweight CPAP/BiPAP machines, wearable oxygen concentrators, and battery-operated nebulizers, improving mobility and patient comfort
- For instance, in February 2024, Philips launched an upgraded version of its portable BiPAP device with enhanced connectivity features for real-time monitoring and telemedicine support
- This trend aligns with the broader shift toward personalized care, remote patient monitoring, and reduced hospital readmissions, especially in countries with advanced healthcare systems like Germany, France, and the UK.
Anesthesia and Respiratory Devices Market Dynamics
Driver
“Growing Burden of Chronic Respiratory Diseases and Surgical Procedures”
- The increasing prevalence of chronic respiratory conditions such as COPD, asthma, and sleep apnea is significantly driving demand for respiratory support devices across Europe
- Moreover, the rising number of surgeries, especially in aging populations, is boosting the need for reliable and efficient anesthesia delivery systems
- For example, according to Eurostat 2023 data, the number of surgical procedures performed in European hospitals rose by over 8% year-over-year, with the largest increases in orthopedic and cardiovascular surgeries
- The continuous advancements in ventilator technology, anesthesia monitoring, and integration with electronic health records (EHRs) are further strengthening market adoption
Opportunity
“Adoption of Smart, AI-Powered Anesthesia and Respiratory Devices”
- The European healthcare sector is increasingly embracing AI-integrated devices that enhance precision in respiratory therapy and anesthesia delivery through real-time data analytics and automation
- Smart ventilators and anesthesia machines now feature adaptive ventilation modes, auto-titration, and remote diagnostics, making them ideal for ICU and home care settings
- In April 2024, GE HealthCare introduced an AI-powered anesthesia system in select European hospitals, offering predictive insights for dose adjustments and improving patient safety
- This digital transformation opens new avenues for manufacturers to offer value-added solutions aligned with the region's growing emphasis on smart hospitals and connected care ecosystems
Restraint/Challenge
“Stringent Regulatory Requirements and Cost Constraints”
- The European medical device market is governed by rigorous regulatory frameworks such as the Medical Device Regulation (MDR), which increases time-to-market and compliance costs for manufacturers
- High capital investment in advanced anesthesia and respiratory devices often limits adoption in small and mid-sized healthcare facilities, particularly in Central and Eastern Europe
- According to a 2023 MedTech Europe report, the average regulatory compliance cost for a new Class II respiratory device increased by 35% under MDR compared to previous directives
- Additionally, limited reimbursement coverage for home-care respiratory devices in certain countries restricts broader access to innovative technologies, creating disparities in market penetration across the region
Anesthesia and Respiratory Devices Market Scope
The market is segmented on the basis of product, raw material, and application.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By End User |
|
Anesthesia and Respiratory Devices Market Regional Analysis
“Germany is the Dominant Region in the Anesthesia and Respiratory Devices Market”
- Germany leads the Anesthesia and Respiratory Devices Market, supported by its highly developed healthcare infrastructure, strong regulatory framework, and rising prevalence of respiratory and chronic diseases
- The region has a large aging population, especially in countries like Germany, France, Italy, and the UK, driving the demand for advanced respiratory support and anesthesia systems in both hospitals and home care settings
- European governments and private healthcare providers are increasingly investing in cutting-edge medical devices to improve patient outcomes and reduce hospital stays
- Furthermore, the presence of leading global medical technology companies and a strong focus on medical innovation and digital health solutions further strengthens Europe’s position as the dominant regional market
“France is Projected to Register the Highest Growth Rate”
- France is expected to witness the fastest growth in the Anesthesia and Respiratory Devices Market, fueled by rapid healthcare infrastructure development, increasing healthcare expenditure, and rising awareness about respiratory health.
- The surge in cases of chronic respiratory conditions, urban pollution, and a growing middle-class population are boosting the demand for both portable and high-end respiratory and anesthesia devices.
- Additionally, local manufacturing, favorable government initiatives, and entry of global players into regional markets are expected to significantly drive growth across the country.
Anesthesia and Respiratory Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Anesthesia and Respiratory Devices Market Regional Analysis
Germany Anesthesia and Respiratory Devices Market Insight
Germany led the Europe anesthesia and respiratory devices market with a revenue share of 24.67% in 2024, supported by a mature healthcare infrastructure, high surgical volumes, and innovation in ventilation and anesthesia monitoring systems. The presence of global leaders like Drägerwerk and B. Braun fosters technological adoption.
Germany is expected to maintain its leadership, driven by government investments in intensive care capacity and digital health transformation. Rising demand for minimally invasive anesthesia and integrated respiratory solutions supports continued market expansion.
U.K. Anesthesia and Respiratory Devices Market Insight
The United Kingdom is poised to grow at the fastest CAGR of 9.11% from 2025 to 2032, owing to a strong focus on digital health, increased ICU capacity, and post-pandemic respiratory care needs. The U.K. accounted for 21.03% of the market in 2024.
Rapid adoption of AI-enabled respiratory monitoring and smart anesthesia workstations, supported by NHS modernization and R&D initiatives, positions the U.K. as a key growth driver in the European market.
France Anesthesia and Respiratory Devices Market Insight
France held a notable market share of 19.58% in 2024, driven by increased surgical procedures, aging population, and focus on perioperative care optimization. Strong hospital infrastructure in cities like Lyon and Toulouse supports steady market demand.
France’s market is projected to grow at a significant CAGR, fueled by strategic procurement of next-gen ventilators and eco-friendly anesthesia machines. Partnerships between public hospitals and device manufacturers bolster technology integration.
The Major Market Leaders Operating in the Market Are:
- Philips Healthcare (Netherlands)
- GE HealthCare (U.S)
- Medtronic plc (Ireland)
- Drägerwerk AG & Co. KGaA (Germany)
- Fisher & Paykel Healthcare Corporation Limited (New Zealand)
- Smiths Medical (ICU Medical, Inc.) (U.S)
- Getinge AB (Sweden)
- Hamilton Medical AG (Switzerland)
- Masimo Corporation (U.S)
- Nihon Kohden Corporation (Japan)
- ResMed Inc. (U.S)
- Mindray Medical International Limited (China)
- Koninklijke Philips N.V. (Netherlands)
- Teleflex Incorporated (U.S)
- Vyaire Medical, Inc. (U.S)
- Schiller AG (Switzerland)
- Siare Engineering International Group (Italy)
- Beurer GmbH (Germany)
- Weinmann Emergency Medical Technology GmbH (Germany)
Latest Developments in Europe Anesthesia and Respiratory Devices Market
- In February 2024, Philips Healthcare introduced a next-generation portable BiPAP device featuring integrated AI algorithms for automatic pressure adjustment, targeted at home-care and sleep apnea management across Western Europe
- In January 2024, GE HealthCare expanded its portfolio with a smart anesthesia delivery system equipped with predictive analytics, aimed at improving precision dosing in surgical environments in Germany and France
- In October 2023, Drägerwerk AG launched a new line of compact ventilators tailored for ambulatory and transport use, addressing growing demand for emergency and mobile respiratory care in the UK and Nordic countries
- In August 2023, Hamilton Medical AG announced the launch of a cloud-enabled ventilator management platform across Europe, improving remote monitoring capabilities in ICU settings
- In June 2023, Vyaire Medical partnered with leading hospitals in Italy and Spain to pilot its automated weaning system for mechanical ventilation, aimed at reducing ICU stays and improving patient recovery times.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.