Europe Biomarkers Market
Market Size in USD Billion
CAGR :
% 
 USD
18.18 Billion
USD
50.41 Billion
2024
2032
USD
18.18 Billion
USD
50.41 Billion
2024
2032
| 2025 –2032 | |
| USD 18.18 Billion | |
| USD 50.41 Billion | |
|
|
|
|
Europe Biomarkers Market Size
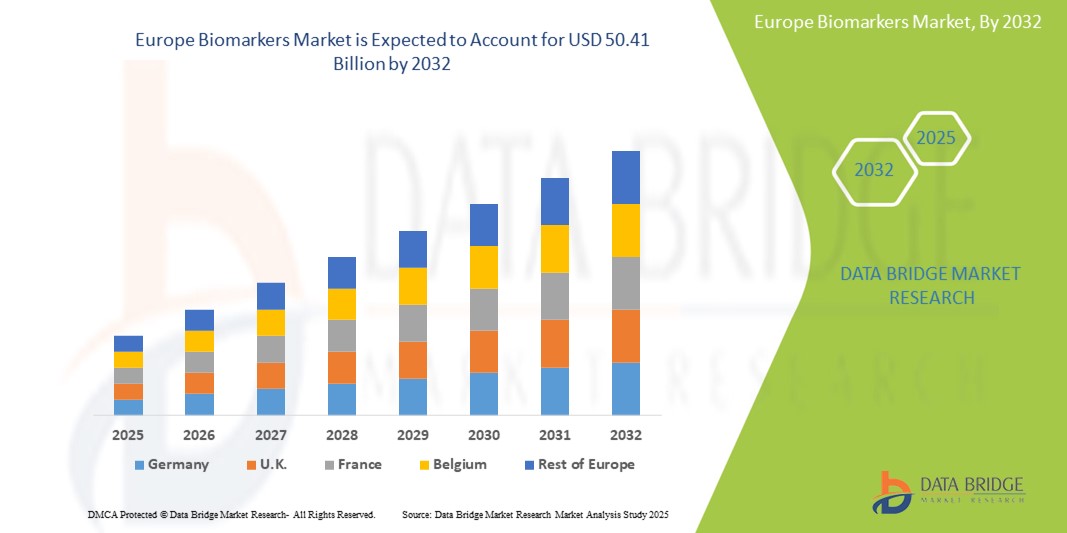
- The Europe biomarkers market size was valued at USD 18.18 billion in 2024 and is expected to reach USD 50.41 billion by 2032, at a CAGR of 13.60% during the forecast period
- This growth is driven by factors such as the increasing prevalence of chronic diseases, advancements in personalized medicine, the growing demand for early diagnosis, the rising use of biomarkers in drug development and clinical trials, and the expansion of biotechnology and pharmaceutical industries
Europe Biomarkers Market Analysis
- The Europe biomarkers market is witnessing significant growth due to the rising demand for precise diagnostics, with biomarkers playing a critical role in early detection and monitoring of various diseases
- Technological advancements in biomarker discovery are revolutionizing the market, with innovative platforms accelerating the identification of novel biomarkers for different therapeutic areas
- Germany is expected to dominate the biomarkers market with 12.3% market share due to its robust healthcare system facilitates the adoption of innovative diagnostic technologies, including biomarkers, into clinical practice
- France is expected to be the fastest growing region in the Europe biomarkers market with 11.6% market share during the forecast period due to the increasing demand for personalized medicine, advancements in diagnostic technologies, and a growing emphasis on early disease detection
- The safety biomarkers segment is expected to dominate the Europe biomarkers market with the largest share of 49.05% in 2025 due to its critical role in assessing drug safety during development. These biomarkers help identify potential adverse effects early, reducing the risk of late-stage clinical trial failures. Their application enhances patient safety by predicting toxicological responses, leading to more effective and safer therapeutic interventions. The increasing prevalence of chronic diseases and the demand for personalized medicine further drive the adoption of safety biomarkers
Report Scope and Europe Biomarkers Market Segmentation
|
Attributes |
Europe Biomarkers Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Europe Biomarkers Market Trends
"Growth of Personalized Medicine in Biomarkers Market"
- The Europe biomarkers market is growing significantly due to the rising demand for personalized medicine, which customizes treatments based on individual genetic profiles. This trend is improving the effectiveness of treatments and reducing adverse reactions for patients
- Advancements in technologies such as next-generation sequencing and liquid biopsies are enhancing biomarker discovery. These innovations allow for more accurate and less invasive methods of detecting biomarkers associated with various diseases, contributing to better diagnosis
- Regulatory support for personalized medicine is a key factor in the market’s expansion. Authorities are encouraging the development of companion diagnostics, ensuring that targeted therapies can be used safely and effectively in personalized treatment plans
- The growing focus on patient-centered care is also fueling the biomarkers market. Healthcare systems are increasingly adopting personalized approaches, emphasizing the need for specific biomarkers to better understand and treat individual patient conditions
- For instance, artificial intelligence is being integrated into the biomarker discovery process. This technology is improving the speed and accuracy of identifying new biomarkers, accelerating the development of more precise and effective personalized treatments
- In conclusion, overall, the growing emphasis on personalized medicine and technological advancements is driving the expansion of the biomarkers market
Europe Biomarkers Market Dynamics
Driver
“Increasing Prevalence of Chronic Diseases”
- The rising prevalence of chronic diseases in Europe, including cancer, cardiovascular diseases, and diabetes, is accelerating the demand for biomarkers. The aging population and lifestyle changes are contributing to the growing need for early diagnosis and personalized treatments
- Biomarkers are becoming essential for early detection and monitoring of chronic diseases. For example, cancer biomarkers are used to identify tumor types, enabling doctors to select the most effective treatment strategies
- Personalized medicine, guided by biomarkers, is improving treatment outcomes by offering more tailored solutions. Biomarkers help to minimize adverse reactions by matching patients with the most appropriate therapies based on their unique genetic profiles
- The increasing demand for early detection is fueling the growth of biomarker-based diagnostics. For instance, biomarkers are vital in screening for cancers, allowing for earlier intervention and improved survival rates
- As awareness of chronic diseases grows, more healthcare providers are integrating biomarkers into their diagnostic and treatment processes. This is leading to greater investment in biomarker research and development across Europe
Opportunity
“Integration of Artificial Intelligence in Biomarker Discovery”
- Artificial intelligence is transforming biomarker discovery by efficiently analyzing vast clinical datasets and genetic information to identify new biomarkers. This allows for more accurate and faster identification compared to traditional methods
- AI's capability to process complex data enables the discovery of rare biomarkers, especially for diseases such as cancer and neurological disorders. This increases the such aslihood of detecting previously overlooked patterns
- Machine learning tools driven by AI are enhancing personalized medicine by predicting how patients will respond to specific treatments. This ensures patients receive the most effective and tailored therapy
- For instance, AI is used in precision oncology to match patients with treatments based on their genetic profiles, improving treatment outcomes and reducing unnecessary therapies
- The increasing adoption of AI technologies in healthcare provides a major opportunity for further advancements in biomarker research. As AI tools evolve, they will drive innovation in diagnostics and treatment options
- In conclusion, overall, the integration of AI in biomarker discovery is set to revolutionize healthcare by accelerating diagnostics and improving patient care
Restraint/Challenge
“High Cost of Biomarker Development”
- The high cost of developing and commercializing biomarkers is a major challenge in the market, requiring significant investments in research, clinical trials, and technology. The process of discovering and validating biomarkers can take years and involves substantial financial resources
- Extensive testing, data analysis, and clinical validation make the development of reliable biomarkers an expensive process. This burden is particularly heavy for smaller biotech companies with limited funding, making it difficult to compete with larger, more resource-rich organizations
- Regulatory hurdles for biomarker-based products also contribute to high costs, as stringent approval processes require additional investments. These delays in market entry further increase the financial burden on companies developing biomarker-based diagnostics
- The cost of biomarker development is often passed onto healthcare systems and patients, which can limit the adoption of biomarker-based diagnostics. This is particularly problematic in low- and middle-income regions, where access to advanced diagnostics may be limited
Europe Biomarkers Market Scope
The market is segmented on the basis of type, product, mechanism, application, and disease indication.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Product |
|
|
By Mechanism |
|
|
By Application
|
|
|
By Disease Indication |
|
In 2025, the safety biomarkers segment is projected to dominate the market with a largest share in type segment
The safety biomarkers segment is expected to dominate the Europe biomarkers market with the largest share of 49.05% in 2025 due to its critical role in assessing drug safety during development. These biomarkers help identify potential adverse effects early, reducing the risk of late-stage clinical trial failures. Their application enhances patient safety by predicting toxicological responses, leading to more effective and safer therapeutic interventions. The increasing prevalence of chronic diseases and the demand for personalized medicine further drive the adoption of safety biomarkers.
The diagnostics development segment is expected to account for the largest share during the forecast period in application segment
In 2025, the diagnostics development segment is expected to dominate the market with the largest market share of 38.05% due to the escalating demand for early and precise disease detection, particularly for conditions such as cancer and neurological disorders. Advancements in diagnostic technologies, including next-generation sequencing (NGS), polymerase chain reaction (PCR), and mass spectrometry, have significantly enhanced the sensitivity and specificity of biomarker-based tests, facilitating more accurate diagnoses. The integration of artificial intelligence (AI) and machine learning (ML) in biomarker discovery has further streamlined data analysis, enabling the identification of complex biomarker patterns and supporting personalized diagnostic approaches.
Europe Biomarkers Market Regional Analysis
“Germany Holds the Largest Share in the Europe Biomarkers Market”
- Germany's robust healthcare system facilitates the adoption of innovative diagnostic technologies, including biomarkers, into clinical practice
- The country hosts a vast network of research institutions, such as the German Cancer Research Center (DKFZ), fostering advancements in genomics and personalized medicine with 12.3% market share
- Government-backed initiatives such as the National Genome Research Network (NGFN) support large-scale genomic projects, enhancing biomarker development
- Germany's regulatory environment, governed by agencies such as the European Medicines Agency (EMA) and the Federal Institute for Drugs and Medical Devices (BfArM), expedites the approval of innovative biomarker-based therapies and diagnostics
- These factors collectively position Germany at the forefront of the personalized medicine biomarkers market in Europe
“France is Projected to Register the Highest CAGR in the Europe Biomarkers Market”
- The France market expansion is due to the increasing demand for personalized medicine, advancements in diagnostic technologies, and a growing emphasis on early disease detection
- Key segments contributing to this growth include safety biomarkers, with efficacy biomarkers emerging as the fastest-growing segment with 11.6% market share during the forecast period
- France's strong healthcare infrastructure, coupled with substantial investments in research and development, supports the rapid adoption of biomarker-based diagnostics and therapies
- The country's regulatory environment facilitates the development and commercialization of innovative biomarker technologies, further accelerating market growth
- Collaborations between pharmaceutical, biotechnology, and diagnostic companies enhance innovation and market growth
Europe Biomarkers Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Abbott (U.S.)
- QIAGEN (Germany)
- PerkinElmer, Inc. (U.S.)
- Merck KGaA (Germany)
- Bio-Rad Laboratories, Inc. (U.S.)
- Enzo Biochem, Inc. (U.S.)
- Charles River Laboratories International, Inc. (U.S.)
- Eurofins Scientific (Luxembourg)
- Agilent Technologies, Inc. (U.S.)
- Bruker (U.S.)
- Siemens (U.S.)
- Epigenomics AG (Germany)
- General Electric (U.S.)
- bioMérieux SA (France)
- Oxford BioDynamics Plc (U.K.)
- Proteome Sciences plc (U.K.)
- Abcam plc (U.K.)
- BiognostiX AG (Switzerland)
Latest Developments in Europe Biomarkers Market
- In May 2024, Genialis introduced a machine learning-based biomarker tool called Genialis krasID, designed to help identify patients most likely to benefit from KRAS inhibitors. This innovative tool also offers an in-depth analysis of response rates and the extent of effectiveness, helping personalize cancer treatment strategies and improve patient outcomes
- In April 2024, Owlstone Medical received a significant grant of USD 1.5 million from the Bill & Melinda Gates Foundation. The funding aims to advance their development of breath-based diagnostics, focusing on the identification of breath biomarkers for diseases such as tuberculosis and human immunodeficiency virus (HIV). This project holds the potential to revolutionize diagnostic approaches for these infectious diseases by providing non-invasive, rapid, and highly accurate testing methods
- In April 2024, Bio-Rad Laboratories launched the ddPLEX ESR1 mutation detection kit, an ultrasensitive and multiplexed digital PCR assay. This kit is designed to detect ESR1 mutations in breast cancer during clinical research, enabling more precise detection and monitoring of treatment efficacy. The advancement of this kit could contribute to more effective management of breast cancer by helping clinicians track mutations that influence treatment decisions
- In March 2024, Koneksa, a company focused on developing evidence-based biomarkers, announced a partnership with Merck through its Data Syndication Partnership Program. The collaboration aims to accelerate the development of digital biomarkers for neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. This initiative is expected to help create innovative tools for early detection, monitoring disease progression, and optimizing treatment strategies, ultimately improving patient care in neurodegenerative diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.