Europe Breast Cancer Diagnostics Market Analysis and Insights
Breast cancer is the most common disease in the population, occurring mainly in women with proliferative diseases. Breast cancer develops mainly in the breast tissues. Symptoms of breast cancer include lumps in the breast, a difference in the size and shape of the breasts, discharge from the nipples, and irritated, scaly red patches on the skin. The incidence of breast cancer in women increases every time the growth of the breast cancer market increases with the growth of drugs. Advanced methods for diagnosis of breast cancer with developed techniques. Improving the technologies developed in breast cancer by increasing the effectiveness, accuracy, efficiency, reliability and speed of results and improving the detection of disorders. Advanced features to detect cancer in less time. Cancer is detected at an early stage, which helps patients recover faster, prevent cancer cells from spreading to other parts of the body, and treat them with new drugs on the market. Increased tumor screening and imaging will drive the breast cancer market. This is expanding the breast cancer market with an increasing number of patients every year, and the rate of recovery from breast cancer is increasing. Government support by increasing the availability of new drug development projects and increasing the effectiveness and efficiency of treatment and the integration of new drugs into the medical field.
However, the high cost of cancer diagnostic treatments and the lack of skilled professionals are the factors that are restraining the market growth.
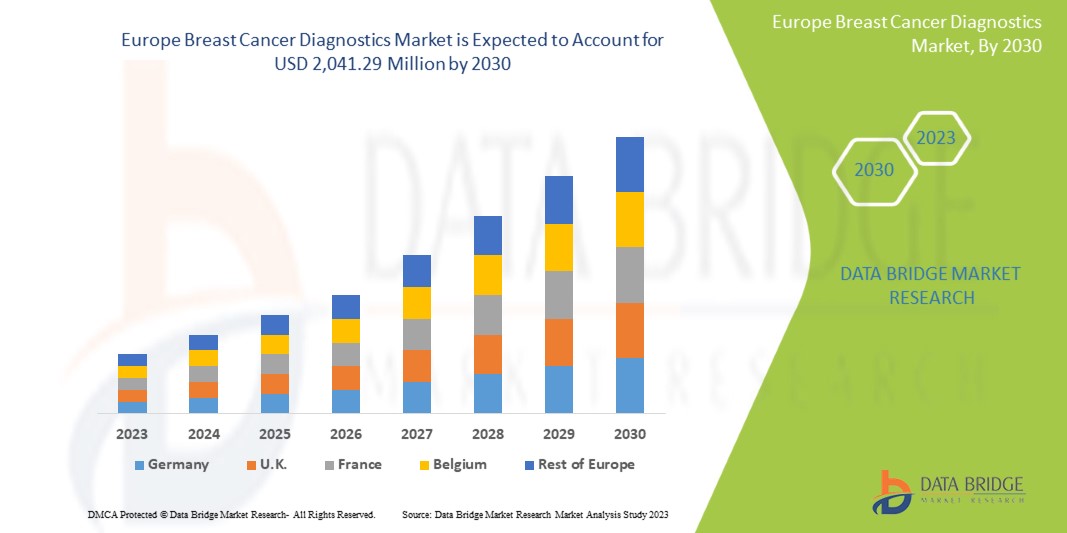
Data Bridge Market Research analyzes that the Europe breast cancer diagnostics market is expected to reach the value of USD 2,041.29 million by 2030, at a CAGR of 8.7% during the forecast period. Test type accounts for the largest diagnostic type segment in the market due to rising breast cancer patients and technological advancements in treatment of breast cancer Europe. This market report also covers pricing analysis, patent analysis, and technological advancements in depth.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2020-2016) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Test Type (Imaging, Biopsy, Genomic Test, Blood Test, and Others), Type (Ductal In Situ Carcinoma, Invasive Ductal Carcinoma, Inflammatory Breast Cancer, and Metastatic Breast Cancer), End User (Hospitals, Clinics, Research and Academic Institutes, Diagnostic Centers, and Others) Distribution Channel (Direct Tender, Retail Sales, and Others). |
|
Countries Covered |
Germany, France, U.K., Italy, Russia, Spain, Netherlands, Switzerland, Belgium, Turkey, Ireland and Rest of Europe. |
|
Market Players Covered |
The major companies which are dealing in the market are F-Hoffmann La Roche Ltd., Siemens Healthcare GmbH, General Electric, Koninklijke Philips N.V., FUJIFILM Corporation, Abbott, Hologic, Inc., OncoStem, Provista Diagnostics, Thermo Fisher Scientific Inc., Myriad Genetics, Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., BD, NanoString., Cepheid, BIOMÉRIEUX, Exact Sciences Corporation, Biocept, Inc., and Abacus ALS, among others. |
Europe Breast Cancer Diagnostics Market Definition
Breast cancer is a disease in which the uncontrolled growth of malignant cells in the breast tissue occurs more often in women than in men. Breast cancer is the uncontrolled cell division of breast cells, which are the most common cells in the mammary glands and ducts. Some symptoms of breast cancer include a lump or lump in the breast, bloody discharge from the nipple and a change in the shape of the nipple or breast. Breast cancer treatment depends on the stage of cancer. Its treatment consists of chemotherapy, radiation therapy, hormone therapy, and surgery.
Breast cancer is another type of cancer that is more common in women than in men. A number of the symptoms of the sickness hold bleeding secretion from the reproductive organ, a lump or block within the breast, and variations in the texture or form of the breast or nipple. Breast cancer treatment depends on the stage of cancer. In addition, its treatment includes radiotherapy, therapy, hormone therapy and surgery. Early detection of carcinoma is essential for the effective treatment of the disease. Early detection of the disease also leads to better outcomes, including many treatment options, better survival and better quality of life. Increasing pressure and demand for new forms of treatment or methods of treatment are due to the rapidly increasing spread of diseases.
Europe Breast Cancer Diagnostics Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Launch of technologically advanced diagnosis & therapeutics to aid growth
Breast cancer is the most common disease in the population, occurring mainly in women with proliferative disease. Breast cancer develops in the breast tissues. Symptoms of breast cancer include lumps in the breast, differences in the size and shape of the breast, discharge from the nipples, and itchy, scaly red patches on the skin. This disease spreads in body with increasing symptoms such as bone pain, swollen lymph nodes, difficulty in breathing and many others. Increasing research and development with new technologies and treatments developed for breast cancer will significantly contribute to market growth.
Moreover, Technological advances in imaging are creating new opportunities for improvements in both screening and early detection. One technology advance is 3-D mammography, also called breast tomosynthesis. This procedure takes images from different angles around the breast and builds them into a 3-D-like image.
In the recent times, several advancements in technology is changing the fate of cancer diagnostics, as mentioned above some of the recent advancements, several other technologies are currently under trial phase which are on the limit of changing the process of breast cancer diagnostics. These technological advancements in the field are acting as a major drivers for Europe breast cancer diagnostics market.
- Increasing prevalence of breast cancer
One of the major factors driving the growth of the Europe market is the increasing prevalence of breast cancer worldwide, which is expected to lead to a large number of patients in need of accurate and effective treatment options. Breast cancer is one of the most common cancers in the world, and better diagnostics are expected to increase the number of patients diagnosed in the future.
In addition, the growing demand for preventive care and early treatment has led to many cancer screenings, which would also increase the number of patients with a high need for treatment during the forecast period.
Thus, increasing prevalence of breast cancer leads to more diagnosis of cases and hence it is expected to drive the market growth during the forecast period.
Restraint
- Adverse effects of breast cancer therapeutics
Screening is looking for cancer before a person has any symptoms. This can help find cancer at an early stage. When abnormal tissue or cancer is found early, it may be easier to treat. By the time symptoms appear, cancer may have begun to spread. Several screening tests are used for detecting breast cancers which include breast magnetic resonance imaging, diagnostic mammogram, biopsy etc. Although these test are considered to be standard for diagnosing breast cancer, they have their associated risks which include false-negative results, false-positive results, and side effects such as undue psychological stress, excessive radiation exposure, and a serious risk of tumor rupture and spread of cancerous cells.
Opportunity
- Initiatives taken by the government
Government is focusing on increasing the initiative for breast cancer treatments with advanced techniques to improve the health of patients. Providing he number of insurances, such as benefits, has been increased so that ordinary people can choose treatment and take advantage of available medicines and treatments to improve their quality of life. In developing countries such as China and India, the government has launched several programs and initiatives to raise awareness about the disease. This has greatly increased the demand for breast cancer diagnosis and disease management therapies. Increasing mergers and acquisitions between pharmaceutical companies and government agencies are among the main trends in the Europe breast cancer market.
Rates of new breast cancer cases have risen 0.6% per year from 2010-2019, and death rates have risen an average of 1.7% per year for the same time frame. As the breast cancer patient rate is increasing, the use of treatments and advanced technologies for the diagnosis of cancer will increase, which is an opportunity for market growth.
Challenge
- Lack of diagnostic infrastructure
Non-communicable diseases such as breast cancer and other types of cancer have now been recognized by the United Nations and WHO as a major public health crisis. Cancer is the biggest part of this problem, and health systems face a major challenge to improve cancer care, control costs and increase system efficiency. Differences in treatment approach and outcomes between high income countries and low and middle income countries are striking. Reasons for this disparity include cost, access to care, workforce and training gaps, and lack of awareness among the lay and medical community.
Moreover, compared to early diagnosis, cancer screening is a separate and more complex public health strategy that requires additional resources, infrastructure and coordination. Rural, developing and low income countries in the different regions does not have enough infrastructure to maintain with the new kits and fro the sample storage as well. These countries need diagnostic infrastructure to ensure the early detection and rapid, accurate diagnosis of the disease.
Post-COVID-19 Impact on Europe Breast Cancer Diagnostics Market
The COVID-19 pandemic has caused disruptions in elective health services related to breast screening, pre-cancer care and management of abnormal screening results. This could lead to an increase in the incidence of breast cancer, exacerbating existing health inequalities.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities and product launch and strategic partnerships to improve the technology and test results involved in the market.
Recent Developments
- In November 2022, Koninklijke Philips N.V., announced the Europe launch of a next-generation compact portable ultrasound solution at the Radiological Society of North America (RSNA) annual meeting to bring the diagnostic quality associated with premium cart-based ultrasound systems to more patients. It is portable and versatile with good image quality or performance. It is Compatible with Philips ultrasound systems Affiniti and EPIQ transducer. This has helped company to expand its product portfolio.
- In November 2022, Siemens Healthineers and Atrium Health, a leading not-for-profit healthcare provider known for leading pediatric, cancer and cardiac care programs, have announced a multi-year value partnership1. This strategic agreement focuses on improving access to care in Atrium Health's service area in the southeastern United States, improving health equity and increasing economic mobility. Atrium Health is acquiring more than $140 million in equipment and devices from Siemens Healthineers, including advanced imaging technology, radiation oncology and precision endovascular robotics. This has helped the company to expand their business.
Europe Breast Cancer Diagnostics Market Scope
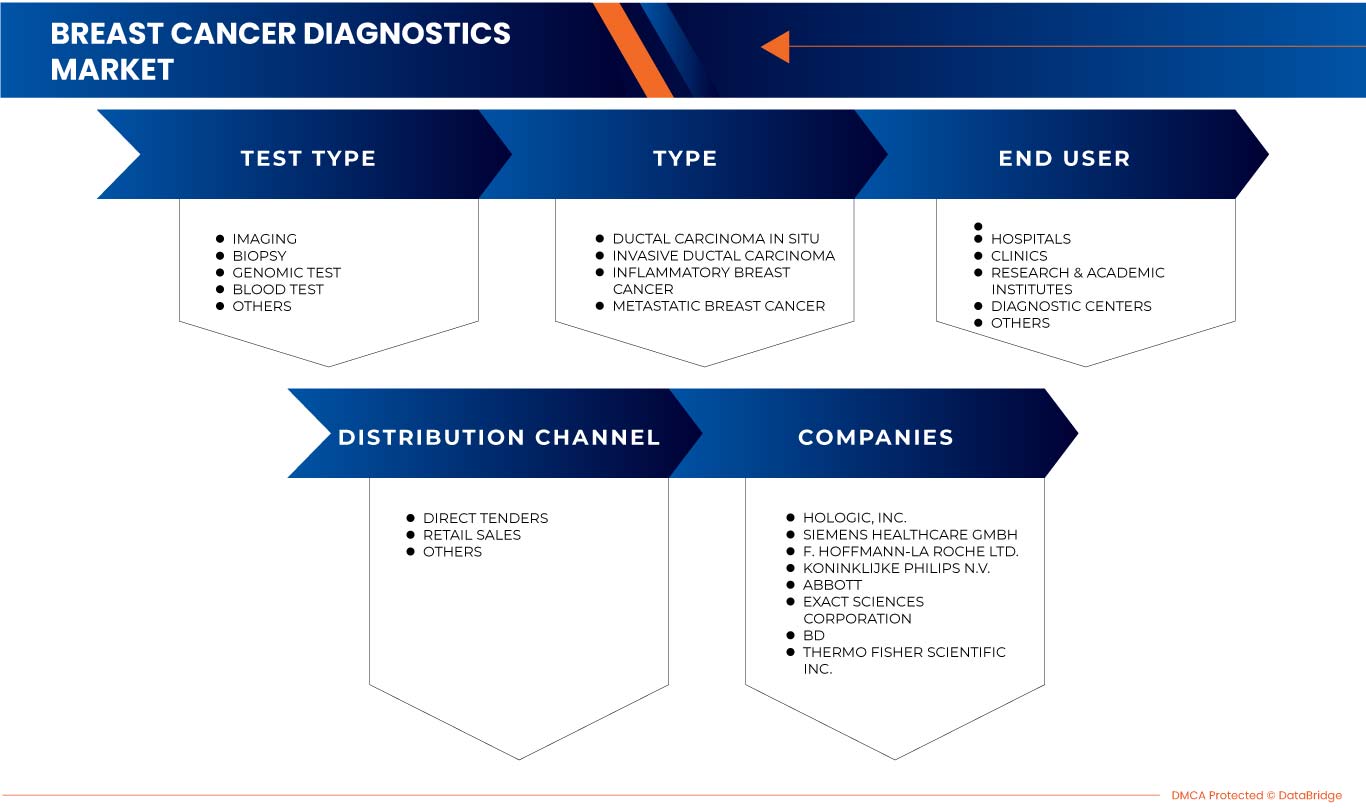
Europe breast cancer diagnostics market is segmented into test type, type, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE
- IMAGING
- BIOPSY
- GENOMIC TEST
- BLOOD TEST
- OTHERS
On the basis of test type, the Europe breast cancer diagnostic market is segmented into imaging, biopsy, genomic test, blood tests and others.
EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE
- DUCTAL CARCINOMA IN SITU
- INVASIVE DUCTAL CARCINOMA
- INFLAMMATORY BREAST CANCER
- METASTATIC BREAST CANCER
On the basis of type, the Europe breast cancer diagnostic market is segmented into ductal in situ carcinoma, invasive ductal carcinoma, inflammatory breast cancer, and metastatic breast cancer.
EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY END USER
- HOSPITALS
- CLINICS
- RESEARCH & ACADEMIC INSTITUTES
- DIAGNOSTIC CENTERS
- OTHERS
On the basis of end user, the Europe breast cancer diagnostic market is segmented into hospitals, clinics, research and academic institutes, diagnostic centers and others.
EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
- DIRECT TENDER
- RETAIL SALES
- OTHERS
On the basis of distribution channel, the Europe breast cancer diagnostic market is segmented into direct tender, retail sales and others.
Europe Breast Cancer Diagnostics Market Regional Analysis/Insights
The Europe breast cancer diagnostics market is analyzed and market size information is provided test type, type, end user, and distribution channel.
The countries covered in this market report Germany, France, U.K., Italy, Russia, Spain, Netherlands, Switzerland, Belgium, Turkey, Ireland and rest of Europe.
In 2023, U.K. dominates Europe region due to the mass production of breast cancer diagnostics drugs and increasing demand from emerging markets and expansion of cancer treatment industries.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Breast Cancer Diagnostics Market Share Analysis
Europe breast cancer diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Europe breast cancer diagnostics market.
Some of the major players operating in the Europe breast cancer diagnostics market are F-Hoffmann La Roche Ltd., Siemens Healthcare GmbH, General Electric, Koninklijke Philips N.V., FUJIFILM Corporation, Abbott, Hologic, Inc., OncoStem, Provista Diagnostics, Thermo Fisher Scientific Inc., Myriad Genetics, Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., BD, NanoString., Cepheid, BIOMÉRIEUX, Exact Sciences Corporation, Biocept, Inc., and Abacus ALS.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE BREAST CANCER DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 EPIDEMIOLOGY
6 INDUSTRIAL INSIGHTS
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 LAUNCH OF TECHNOLOGICALLY ADVANCED DIAGNOSIS & THERAPEUTICS TO AID GROWTH
8.1.2 INCREASING PREVALENCE OF BREAST CANCER
8.1.3 GROWING IMPORTANCE OF WOMEN'S HEALTH
8.1.4 RISING AWARENESS TOWARDS THE BREAST CANCER
8.2 RESTRAINTS
8.2.1 ADVERSE EFFECTS OF BREAST CANCER THERAPEUTICS
8.2.2 HIGH COST OF THE IMAGING SYSTEMS
8.3 OPPORTUNITIES
8.3.1 INITIATIVES TAKEN BY THE GOVERNMENT
8.3.2 STRATEGIC INITIATIVES TAKEN BY THE KEY MARKET PLAYERS
8.3.3 GROWTH IN RESEARCH AND DEVELOPMENT OF BREAST CANCER
8.4 CHALLENGES
8.4.1 LACK OF DIAGNOSTIC INFRASTRUCTURE
8.4.2 LACK OF SKILLED PROFESSIONALS FOR PROPER DIAGNOSIS OF BREAST CANCER
9 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING
9.2.1 IONIZING BREAST IMAGING TECHNOLOGIES
9.2.1.1 FULL-FIELD DIGITAL MAMMOGRAPHY (FFDM)
9.2.1.2 ANALOG MAMMOGRAPHY
9.2.1.3 3D BREAST TOMOSYNTHESIS
9.2.1.4 POSITRON EMISSION TOMOGRAPHY/ COMPUTED TOMOGRAPHY (PET/CT)
9.2.1.5 MOLECULAR BREAST IMAGING/ BREAST SPECIFIC GAMMA IMAGING (MBI/BSMI)
9.2.1.6 POSITRON EMISSION MAMMOGRAPHY
9.2.1.7 OTHERS
9.2.2 NON-IONIZING IMAGING TECHNOLOGIES
9.2.2.1 OPTICAL IMAGING
9.2.2.2 BREAST ULTRASOUND
9.2.2.3 BREAST MRI (MAGNETIC RESONANCE IMAGING)
9.2.2.4 AUTOMATED WHOLE BREAST ULTRASOUND (AWBU)
9.2.2.5 BREAST THERMOGRAPHY
9.2.3 BIOPSY
9.2.3.1 SURGICAL BIOPSY
9.2.3.2 FINE NEEDLE ASPIRATION BIOPSY
9.2.3.3 CORE NEEDLE BIOPSY
9.2.3.4 IMAGE-GUIDED BIOPSY
9.2.3.5 SENTINEL LYMPH NODE BIOPSY
9.2.4 GENOMIC TEST
9.2.4.1 MOLECULAR TESTING
9.2.4.1.1 PD-L1
9.2.4.1.2 MICROSATELLITE INSTABILITY-HIGH (MSI-H) OR DNA MISMATCH REPAIR DEFICIENCY (DMMR)
9.2.4.1.3 NTRK GENE FUSIONS
9.2.4.1.4 PI3KCA GENE MUTATION
9.2.4.2 MAMMAPRINT
9.2.4.3 ONCOTYPE DX
9.2.4.4 OTHERS
9.3 BLOOD TEST
9.4 OTHERS
10 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE
10.1 OVERVIEW
10.2 INVASIVE DUCTAL CARCINOMA
10.3 DUCTAL CARCINOMA IN SITU
10.4 INFLAMMATORY BREAST CANCER
10.5 METASTATIC BREAST CANCER
11 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 DIAGNOSTICS CENTERS
11.4 CLINICS
11.5 RESEARCH & ACADEMIC INSTITUTES
11.6 OTHERS
12 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
12.4 OTHERS
13 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 RUSSIA
13.1.4 ITALY
13.1.5 U.K.
13.1.6 SPAIN
13.1.7 TURKEY
13.1.8 NETHERLANDS
13.1.9 BELGIUM
13.1.10 SWITZERLAND
13.1.11 IRELAND
13.1.12 REST OF EUROPE
14 EUROPE BREAST CANCER DIAGNOSTICS MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 HOLOGIC, INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 SIEMENS HEALTHCARE GMBH
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 F. HOFFMANN- LA ROCHE LTD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 KONINKLIJKE PHILIPS N.V.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 ABBOTT
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 ABACUS ALS
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 BD
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 COMPANY SHARE ANALYSIS
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPMENTS
16.8 BIOCEPT, INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 BIOMÉRIEUX
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 BIO-RAD LABORATORIES, INC.
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 EXACT SCIENCES CORPORATION
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 COMPANY SHARE ANALYSIS
16.12.4 PRODUCT PORTFOLIO
16.12.5 RECENT DEVELOPMENTS
16.13 FUJIFILM CORPORATION
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 GENERAL ELECTRIC
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 ILLUMINA, INC.
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 MYRIAD GENETICS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENT
16.17 NANOSTRING.
16.17.1 COMPANY SNAPSHOT
16.17.2 REVENUE ANALYSIS
16.17.3 PRODUCT PORTFOLIO
16.17.4 RECENT DEVELOPMENT
16.17.5 RECENT DEVELOPMENT
16.18 ONCOSTEM.
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENT
16.19 PROVISTA DIAGNOSTICS.
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 THERMO FISHER SCIENTIFIC INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
List of Table
TABLE 1 INCIDENCE RATE OF BREAST CANCER (2020)
TABLE 2 MORTALITY RATE OF BREAST CANCER (2020)
TABLE 3 INCIDENCE OF DUCTAL CARCINOMA IN SITU (DCIS) BREAST CANCER
TABLE 4 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 EUROPE IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 EUROPE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 EUROPE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 9 EUROPE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 EUROPE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 11 EUROPE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 EUROPE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 14 EUROPE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 EUROPE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 EUROPE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 17 EUROPE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 18 EUROPE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 19 EUROPE BLOOD TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 EUROPE OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 22 EUROPE INVASIVE DUCTAL CARCINOMA IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 EUROPE DUCTAL CARCINOMA IN SITU IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 EUROPE INFLAMMATORY BREAST CANCER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 EUROPE METASTATIC BREAST CANCER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 27 EUROPE HOSPITALS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 EUROPE DIAGNOSTIC CENTERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 EUROPE CLINICS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 EUROPE RESEARCH AND ACADEMIC INSTITUTES IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 EUROPE OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 33 EUROPE DIRECT TENDER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 EUROPE RETAIL SALES IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 EUROPE OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 37 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 38 EUROPE IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 39 EUROPE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 40 EUROPE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 41 EUROPE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 42 EUROPE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 43 EUROPE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 44 EUROPE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 45 EUROPE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 46 EUROPE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 47 EUROPE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 48 EUROPE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 49 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 51 EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 GERMANY BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 53 GERMANY IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 54 GERMANY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 55 GERMANY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 56 GERMANY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 57 GERMANY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 58 GERMANY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 59 GERMANY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 60 GERMANY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 61 GERMANY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 62 GERMANY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 63 GERMANY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 GERMANY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 65 GERMANY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 66 GERMANY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 GERMANY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 68 GERMANY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 69 GERMANY BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 GERMANY BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 GERMANY BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 72 FRANCE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 75 FRANCE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 76 FRANCE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 77 FRANCE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 78 FRANCE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 79 FRANCE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 80 FRANCE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 81 FRANCE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 82 FRANCE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 83 FRANCE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 84 FRANCE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 85 FRANCE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 86 FRANCE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 FRANCE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 88 FRANCE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 89 FRANCE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 90 FRANCE BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 FRANCE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 92 RUSSIA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 93 RUSSIA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 94 RUSSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 95 RUSSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 96 RUSSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 97 RUSSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 98 RUSSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 99 RUSSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 100 RUSSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 RUSSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 102 RUSSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 103 RUSSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 RUSSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 105 RUSSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 106 RUSSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 RUSSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 108 RUSSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 109 RUSSIA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 RUSSIA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 RUSSIA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 112 ITALY BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 ITALY IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 114 ITALY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 115 ITALY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 116 ITALY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 117 ITALY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 118 ITALY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 119 ITALY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 120 ITALY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 121 ITALY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 122 ITALY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 123 ITALY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 124 ITALY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 125 ITALY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 126 ITALY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 127 ITALY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 128 ITALY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 129 ITALY BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 ITALY BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 131 ITALY BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 132 U.K. BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 U.K. IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 U.K. IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 U.K. IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 136 U.K. IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 137 U.K. NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 U.K. NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 139 U.K. NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 140 U.K. BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 141 U.K. BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 142 U.K. BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 143 U.K. GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 144 U.K. GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 145 U.K. GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 146 U.K. MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 147 U.K. MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 148 U.K. MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 149 U.K. BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 150 U.K. BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 151 U.K. BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 152 SPAIN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 153 SPAIN IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 154 SPAIN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 155 SPAIN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 156 SPAIN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 157 SPAIN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 SPAIN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 159 SPAIN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 160 SPAIN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 161 SPAIN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 162 SPAIN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 163 SPAIN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 SPAIN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 165 SPAIN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 166 SPAIN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 SPAIN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 168 SPAIN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 169 SPAIN BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 SPAIN BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 171 SPAIN BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 172 TURKEY BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 173 TURKEY IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 174 TURKEY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 175 TURKEY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 176 TURKEY IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 177 TURKEY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 178 TURKEY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 179 TURKEY NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 180 TURKEY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 181 TURKEY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 182 TURKEY BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 183 TURKEY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 184 TURKEY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 185 TURKEY GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 186 TURKEY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 187 TURKEY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 188 TURKEY MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 189 TURKEY BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 TURKEY BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 191 TURKEY BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 192 NETHERLANDS BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 193 NETHERLANDS IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 194 NETHERLANDS IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 195 NETHERLANDS IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 196 NETHERLANDS IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 197 NETHERLANDS NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 198 NETHERLANDS NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 199 NETHERLANDS NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 200 NETHERLANDS BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 201 NETHERLANDS BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 202 NETHERLANDS BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 203 NETHERLANDS GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 204 NETHERLANDS GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 205 NETHERLANDS GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 206 NETHERLANDS MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 207 NETHERLANDS MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 208 NETHERLANDS MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 209 NETHERLANDS BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 210 NETHERLANDS BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 211 NETHERLANDS BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 212 BELGIUM BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 213 BELGIUM IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 214 BELGIUM IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 215 BELGIUM IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 216 BELGIUM IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 217 BELGIUM NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 218 BELGIUM NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 219 BELGIUM NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 220 BELGIUM BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 221 BELGIUM BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 222 BELGIUM BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 223 BELGIUM GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 224 BELGIUM GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 225 BELGIUM GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 226 BELGIUM MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 227 BELGIUM MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 228 BELGIUM MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 229 BELGIUM BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 BELGIUM BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 231 BELGIUM BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 232 SWITZERLAND BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 233 SWITZERLAND IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 234 SWITZERLAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 235 SWITZERLAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 236 SWITZERLAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 237 SWITZERLAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 238 SWITZERLAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 239 SWITZERLAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 240 SWITZERLAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 241 SWITZERLAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 242 SWITZERLAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 243 SWITZERLAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 244 SWITZERLAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 245 SWITZERLAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 246 SWITZERLAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 247 SWITZERLAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 248 SWITZERLAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 249 SWITZERLAND BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 250 SWITZERLAND BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 251 SWITZERLAND BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 252 IRELAND BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 253 IRELAND IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 254 IRELAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 255 IRELAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 256 IRELAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 257 IRELAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 258 IRELAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 259 IRELAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 260 IRELAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 261 IRELAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 262 IRELAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 263 IRELAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 264 IRELAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 265 IRELAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 266 IRELAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 267 IRELAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 268 IRELAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 269 IRELAND BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 270 IRELAND BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 271 IRELAND BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 272 REST OF EUROPE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 EUROPE BREAST CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 EUROPE BREAST CANCER DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE BREAST CANCER DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 EUROPE BREAST CANCER DIAGNOSTIC MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE BREAST CANCER DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE BREAST CANCER DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE BREAST CANCER DIAGNOSTIC MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 EUROPE BREAST CANCER DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE BREAST CANCER DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE BREAST CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF BREAST CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE EUROPE BREAST CANCER DIAGNOSTIC MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE BREAST CANCER DIAGNOSTIC MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE BREAST CANCER DIAGNOSTICS MARKET
FIGURE 14 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 15 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 18 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, 2022
FIGURE 19 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, CAGR (2023-2030)
FIGURE 21 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, LIFELINE CURVE
FIGURE 22 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 23 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 24 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 25 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 26 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 27 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 28 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 29 EUROPE BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 30 EUROPE BREAST CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 31 EUROPE BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 32 EUROPE BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 33 EUROPE BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 34 EUROPE BREAST CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 35 EUROPE BREAST CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.