Europe Cancer Supportive Care Products Market
Market Size in USD Billion
CAGR :
% 
 USD
10.25 Billion
USD
15.32 Billion
2024
2032
USD
10.25 Billion
USD
15.32 Billion
2024
2032
| 2025 –2032 | |
| USD 10.25 Billion | |
| USD 15.32 Billion | |
|
|
|
|
Cancer Supportive Care Products Market Size
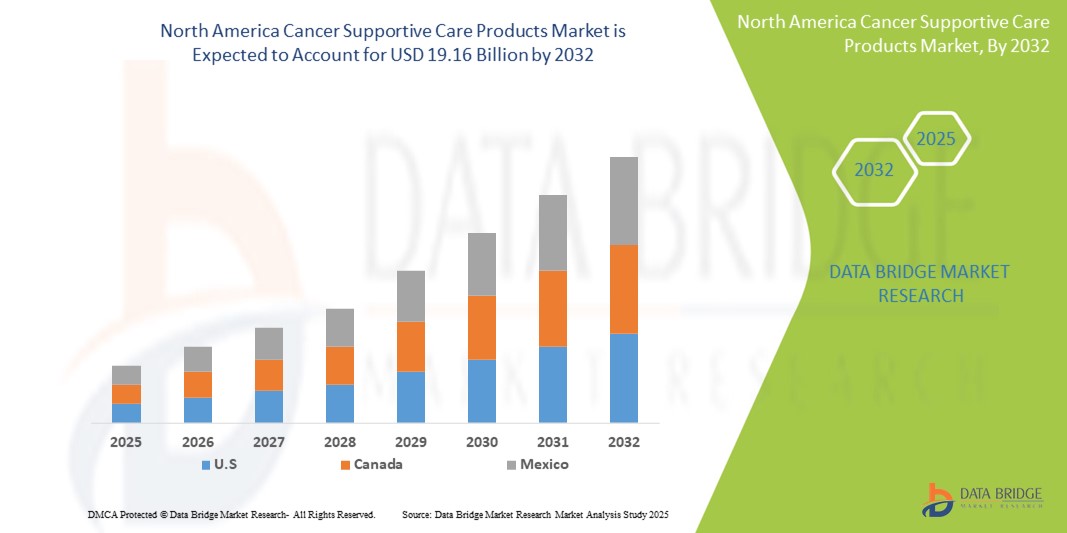
- The Europe Cancer Supportive Care Products Market size was valued at USD 10.25 billion in 2024 and is expected to reach USD 15.32 billion by 2032, at a CAGR of 5.15% during the forecast period
- Market growth is primarily driven by the increasing prevalence of cancer across European countries, coupled with the rising demand for treatments that alleviate the adverse side effects of chemotherapy, radiation, and other oncological interventions
- Moreover, advancements in supportive care drug formulations and the growing availability of targeted supportive therapies through well-established hospital and retail pharmacy networks are enhancing patient compliance and outcomes. These developments are contributing to the sustained expansion of the cancer supportive care products market across Europe
Cancer Supportive Care Products Market Analysis
- Cancer supportive care products, including medications and biologics aimed at mitigating the side effects of cancer treatments, are becoming indispensable in oncology care across Europe. These products enhance quality of life, improve treatment adherence, and reduce hospital readmissions, supporting better overall outcomes for cancer patients.
- The rising incidence of cancer, especially lung, breast, and prostate cancers, coupled with growing awareness about comprehensive cancer management, is significantly driving demand for supportive care therapies such as anti-emetics, granulocyte colony-stimulating factors, erythropoietin-stimulating agents, and monoclonal antibodies.
- Germany dominates the Europe cancer supportive care products market, accounting for the largest revenue share of 29.4% in 2025, supported by its advanced healthcare infrastructure, widespread cancer screening programs, and strong pharmaceutical supply chain.
- The United Kingdom and France are also witnessing substantial market expansion due to increased cancer prevalence, favorable reimbursement policies, and the presence of prominent oncology centers offering supportive care as part of standard treatment protocols.
- The anti-emetics segment is projected to lead the market with a share of 24.6% in 2025, driven by its critical role in managing chemotherapy-induced nausea and vomiting (CINV), thereby improving patient comfort and adherence to chemotherapy regimens.
Report Scope and Cancer Supportive Care Products Market Segmentation
|
Attributes |
Cancer Supportive Care Products Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cancer Supportive Care Products Market Trends
“Advancements in Targeted Supportive Therapies and Biosimilars”
- A prominent trend in the Europe cancer supportive care products market is the increasing adoption of targeted supportive therapies and biosimilars, particularly in managing treatment-related complications such as anemia, neutropenia, and chemotherapy-induced nausea and vomiting (CINV).
- The introduction of biosimilars for key agents like erythropoietin-stimulating agents (ESAs) and granulocyte colony-stimulating factors (G-CSFs) is driving affordability and expanding access to supportive care treatments across the region. For instance, biosimilar versions of filgrastim and epoetin are being increasingly used in public healthcare systems to reduce costs without compromising efficacy.
- European regulatory bodies, especially the European Medicines Agency (EMA), continue to support the development and approval of biosimilars, encouraging pharmaceutical companies to invest in R&D for cost-effective cancer supportive care options.
- Moreover, multimodal supportive care strategies that include both pharmacological and non-pharmacological interventions (such as nutritional support and psychological therapy) are gaining traction in oncology centers across Germany, France, and the UK. These approaches help improve patient adherence to primary treatment regimens and reduce hospitalizations.
- Integration of digital health tools such as symptom tracking apps and telemedicine consultations is also reshaping how supportive care is delivered, enabling real-time monitoring and personalized interventions for better patient outcomes.
Cancer Supportive Care Products Market Dynamics
Driver
“Rising Cancer Burden and Emphasis on Holistic Patient Management”
- The growing incidence of cancer across Europe—particularly breast, lung, prostate, and colorectal cancers—is significantly driving the demand for supportive care solutions that manage the side effects of cancer therapies.
- With over 4 million new cancer cases annually in Europe, there is a heightened need to maintain quality of life and treatment continuity through supportive agents like anti-emetics, opioids, and monoclonal antibodies.
- National cancer control plans in countries like Germany, Italy, and Sweden increasingly emphasize holistic cancer care, which includes pain management, nutritional support, infection prevention, and emotional well-being.
- In 2024, organizations such as the European Society for Medical Oncology (ESMO) pushed forward supportive care guidelines for integration into standard oncology practices, encouraging wider physician adoption.
- The presence of well-developed hospital pharmacy networks and universal healthcare systems in many European nations ensures better accessibility to supportive care medications and helps streamline reimbursement for both branded and biosimilar drugs.
Restraint/Challenge
“Cost Containment Policies and Regional Disparities in Access”
- Despite growth potential, stringent cost-containment measures imposed by European governments and public payers often pose challenges for market expansion, especially for high-cost biologics and newer supportive agents.
- Budget restrictions and the need to prioritize curative therapies may limit the adoption of advanced supportive care drugs in lower-income or rural regions of Eastern and Southern Europe.
- For instance, while Germany and the UK have broad access to G-CSFs and monoclonal antibodies, countries such as Romania and Bulgaria face reimbursement limitations, delaying patient access to optimal supportive care regimens.
- Moreover, variations in clinical practice patterns and physician awareness across regions contribute to inconsistent adoption of comprehensive supportive care strategies.
- Another emerging concern is polypharmacy and drug interactions in cancer patients, especially the elderly population. Managing side effects of supportive agents themselves—such as opioid-induced constipation or ESA-associated cardiovascular risks—adds complexity to treatment planning.
- Addressing these disparities requires collaborative policymaking, improved clinical training, and wider use of cost-effective biosimilars to ensure uniform and equitable access to supportive care products across Europe.
Cancer Supportive Care Products Market Scope
The market is segmented on the basis of drug class, indication, and distribution channel.
• By Drug Class
On the basis of drug class, the Europe cancer supportive care products market is segmented into nonsteroidal anti-inflammatory drugs (NSAIDs), anti-infective, anti-emetics, monoclonal antibodies, erythropoietin-stimulating agents (ESAs), opioid analgesics, bisphosphonates, and granulocyte colony-stimulating factor (G-CSF). The anti-emetics segment dominates the market with the largest revenue share of 24.6% in 2025, primarily due to its critical role in managing chemotherapy-induced nausea and vomiting (CINV). The growing use of 5-HT3 receptor antagonists and NK1 receptor antagonists across oncology departments ensures patient adherence to treatment regimens by minimizing discomfort and enhancing quality of life.
The monoclonal antibodies segment is anticipated to witness the fastest CAGR of 8.9% from 2025 to 2032, driven by increasing use in targeted therapy for managing bone metastases, neutropenia, and other cancer-related complications. As more biosimilar monoclonal antibodies enter the European market, affordability and accessibility are expected to rise, fueling rapid adoption, particularly in public healthcare settings across Western Europe.
• By Indication
On the basis of indication, the market is segmented into lung cancer, breast cancer, prostate cancer, liver cancer, bladder cancer, leukemia, ovary cancer, melanoma cancer, and others. The breast cancer segment accounted for the largest market revenue share in 2024, attributed to its high incidence across Europe and the widespread use of supportive therapies such as anti-emetics, ESAs, and G-CSFs to manage side effects of chemotherapy and hormonal treatments. Countries like France, Germany, and Italy have robust breast cancer screening and treatment programs, further contributing to demand.
The leukemia segment is projected to witness the fastest growth during the forecast period, driven by the complexity of hematologic malignancy treatment protocols and the high requirement for infection prevention, neutropenia management, and transfusion support. Increasing bone marrow and stem cell transplantation procedures also amplify the need for supportive care interventions.
• By Distribution Channel
On the basis of distribution channel, the Europe market is segmented into hospital pharmacies, retail pharmacies, and compounding pharmacies. Hospital pharmacies held the largest market revenue share in 2025, supported by the centralized distribution of cancer therapeutics in institutional settings and the growing preference for in-hospital supportive care management, particularly for high-risk or advanced-stage cancer patients.
The retail pharmacies segment is expected to exhibit the fastest CAGR from 2025 to 2032, driven by increasing availability of supportive care products over-the-counter (e.g., anti-emetics, NSAIDs), growing participation of community pharmacies in chronic care support, and patient preference for convenient local access to prescribed supportive medications.
Cancer Supportive Care Products Market Regional Analysis
- Germany dominates the Europe cancer supportive care products market with the largest revenue share of 29.4%, driven by its advanced oncology infrastructure, high cancer incidence rates, and strong healthcare reimbursement system for supportive care therapies.
- The country’s leadership in clinical oncology is supported by the widespread availability of essential supportive medications such as anti-emetics, erythropoiesis-stimulating agents (ESAs), and granulocyte colony-stimulating factors (G-CSFs), alongside robust diagnostic and treatment frameworks across public and private healthcare institutions.
- Germany also benefits from a high level of investment in cancer research, the presence of major pharmaceutical companies, and continuous introduction of biosimilars and innovative supportive care solutions, further accelerating market growth.
U.K. Cancer Supportive Care Products Market Insight
The U.K. cancer supportive care products market is projected to grow at a steady CAGR during the forecast period, driven by rising cancer prevalence and a strong emphasis on improving patient quality of life throughout treatment. Increased NHS investments in oncology services, along with expanding access to advanced supportive therapies such as anti-emetics, G-CSFs, and opioid analgesics, are key growth drivers. Moreover, the growing awareness around managing chemotherapy side effects and the presence of leading academic medical centers are encouraging early and consistent adoption of supportive care protocols.
Germany Cancer Supportive Care Products Market Insight
The German cancer supportive care products market is expected to expand at a robust CAGR during the forecast period, supported by the country’s advanced healthcare infrastructure, high cancer diagnosis rates, and strong reimbursement mechanisms for supportive therapies. Germany’s commitment to comprehensive oncology care, including access to biosimilars and monoclonal antibodies for managing treatment-related complications, enhances market penetration. In addition, increasing clinical research activity, public-private partnerships, and a proactive regulatory environment are fostering innovation and wider availability of next-generation supportive care products.
Cancer Supportive Care Products Market Share
The Cancer Supportive Care Products industry is primarily led by well-established companies, including:
- Acacia Pharma Group Plc (U.K.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Fagron (Belgium)
- KYOWA HAKKO BIO CO.,LTD. (Japan)
- APR (Switzerland)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Amgen Inc. (U.K.)
- Baxter (U.S.)
- Bayer AG (Germany)
- DAIICHI SANKYO COMPANY, LIMITED. (Japan)
- GSK Plc (U.K.)
Latest Developments in Europe Cancer Supportive Care Products Market
- In June 2023, the FDA approved the use of selumetinib (Koselugo) for treating patients with metastatic melanoma that has progressed after previous therapies. Selumetinib functions as a MEK inhibitor, targeting pathways that drive cancer cell proliferation. This approval expands the treatment options available for late-stage melanoma patients facing limited alternatives
- In May 2023, the FDA granted approval for denosumab (Prolia) to be used in the prevention of bone loss among breast cancer patients receiving aromatase inhibitors. Denosumab, a monoclonal antibody, works by blocking the RANKL protein, which is associated with bone degradation. This decision supports bone health preservation in patients undergoing hormonal cancer therapies
- In April 2023, the FDA authorized the use of pembrolizumab (Keytruda) for the treatment of advanced solid tumors that have progressed despite prior therapies. Pembrolizumab is a checkpoint inhibitor that disrupts the PD-1/PD-L1 interaction, thereby enhancing the immune system's ability to target cancer cells. This broadens the clinical application of immunotherapy in resistant solid tumor cases
- In March 2022, Novartis received FDA approval for Pluvicto, a therapy for metastatic castration-resistant prostate cancer (mCRPC) patients with PSMA-positive tumors who had already undergone hormone and chemotherapy treatments. Pluvicto offers a new avenue of treatment for patients with advanced stages of prostate cancer
- In June 2020, Pfizer Inc. announced that the FDA approved NYVEPRIA (pegfilgrastim-apgf), a biosimilar to Neulasta, designed to reduce the risk of infection due to febrile neutropenia in patients undergoing myelosuppressive chemotherapy for non-myeloid malignancies. This biosimilar expands access to supportive care therapies critical in cancer treatment cycles.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 DRUG TYPE LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 REGULATORY
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 GROWING CANCER BURDEN WORLDWIDE
5.1.2 INCREASING INITIATIVES BY GOVERNMENT AND OTHER HEALTHCARE ORGANIZATIONS
5.1.3 GROWING GERIATRIC POPULATION
5.1.4 RISING NUMBER OF PRODUCT APPROVAL
5.1.5 RISING EXPENDITURE ON HEALTHCARE
5.2 RESTRAINTS
5.2.1 ADVERSE EFFECTS AND RISKS ASSOCIATED WITH CANCER SUPPORTIVE DRUGS
5.2.2 LACK OF EARLY DETECTION
5.3 OPPORTUNITIES
5.3.1 ACQUISITION AND AGREEMENT BY MAJOR PLAYERS
5.3.2 RISING PRODUCT LAUNCHES
5.3.3 GROWING R&D ACTIVITIES
5.4 CHALLENGES
5.4.1 STRINGENT REGULATION POLICY
5.4.2 PATENT EXPIRY OF DRUGS
6 COVID-19 IMPACT ON EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET
6.1 PRICE IMPACT
6.2 IMPACT ON SUPPLY CHAIN
6.3 IMPACT ON DEMAND
6.4 STRATEGIC DECISIONS FOR MANUFACTURERS
6.5 CONCLUSION
7 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE
7.1 OVERVIEW
7.2 GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS)
7.2.1 LONG ACTING FILGRASTIM
7.2.2 FILGRASTIM
7.2.3 LENOGRASTIM
7.3 ERYTHROPOIETIN STIMULATING AGENTS (ESA’S)
7.3.1 EPO-Α/Β
7.3.2 DPO
7.3.3 CERA
7.3.4 EPO-Κ
7.4 OPIOID ANALGESICS
7.4.1 FENTANYL
7.4.2 METHADONE
7.4.3 TRAMADOL
7.4.4 OTHERS
7.5 MONOCLONAL ANTIBODIES
7.6 NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS)
7.6.1 OTC NSAIDS
7.6.1.1 ASPIRIN
7.6.1.2 IBUPROFEN
7.6.1.3 NAPROXEN SODIUM
7.6.2 PRESCRIPTION NSAIDS
7.6.2.1 CELECOXIB
7.6.2.2 DICLOFENAC
7.6.2.3 INDOMETHACIN
7.6.2.4 KETOROLAC
7.6.2.5 MELOXICAM
7.6.2.6 NABUMETONE
7.6.2.7 NAPROXEN
7.6.2.8 OXAPROZIN
7.6.2.9 PIROXICAM
7.6.2.10 SULINDAC
7.6.2.11 OTHERS
7.7 BISPHOSPHONATES
7.7.1 ZOLEDRONIC ACID OR ZOLEDRONATE
7.7.2 DISODIUM PAMIDRONATE
7.7.3 IBANDRONIC ACID OR IBANDRONATE
7.7.4 SODIUM CLODRONATE
7.8 ANTI-EMETICS
7.8.1 APREPITANT
7.8.2 DEXAMETHASONE
7.8.3 DOLASETRON
7.8.4 GRANISETRON
7.8.5 ONDANSETRON
7.8.6 PALONOSETRON
7.8.7 PROCHLORPERAZINE
7.8.8 ROLAPITANT
7.8.9 OTHERS
7.9 ANTIHISTAMINES
7.9.1 HYDROXYZINE
7.9.2 DIPHENHYDRAMINE
7.9.3 OTHERS
7.1 OTHERS
8 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE
8.1 OVERVIEW
8.2 BRANDED
8.2.1 NEULASTA
8.2.2 ARANESP
8.2.3 PROLIA
8.2.4 XGEVA
8.2.5 EPOGEN
8.2.6 EPREX
8.2.7 NEUPOGEN
8.2.8 OTHERS
8.3 GENERICS
9 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE
9.1 OVERVIEW
9.2 LUNG CANCER
9.3 BREAST CANCER
9.4 PROSTATE CANCER
9.5 LIVER CANCER
9.6 BLADDER CANCER
9.7 LEUKAEMIA
9.8 MELANOMA
9.9 OVARIAN CANCER
9.1 OTHER CANCERS
10 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.2.1 ACUTE CARE HOSPITALS
10.2.2 LONG-TERM CARE HOSPITALS
10.2.3 NURSING FACILITIES
10.3 CLINICS
10.4 HOSPITALS & ACADEMIC INSTITUTIONS
10.5 OTHERS
11 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 HOSPITAL PHARMACIES
11.3 RETAIL PHARMACIES
11.4 COMPOUNDING PHARMACIES
12 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY GEOGRAPHY
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 SWITZERLAND
12.1.7 NETHERLANDS
12.1.8 RUSSIA
12.1.9 TURKEY
12.1.10 REST OF EUROPE
13 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 SWOT
15 COMPANY PROFILES
15.1 AMGEN INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 PFIZER INC.
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS
15.3 JANSSEN PHARMACEUTICALS, INC. (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.)
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENTS
15.4 NOVARTIS AG
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 ACACIA PHARMA GROUP PLC
15.5.1 COMPANY SNAPSHOT
15.5.2 PRODUCT PORTFOLIO
15.5.3 RECENT DEVELOPMENTS
15.6 ACROTECH BIOPHARMA
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 APR
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENTS
15.8 BAXTER
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 BAYER AG
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 F. HOFFMANN-LA ROCHE LTD
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENTS
15.11 HELSINN HEALTHCARE SA
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENTS
15.12 HERON THERAPEUTICS, INC.
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 KYOWA KIRIN CO., LTD.
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 MYLAN N.V.
15.15.1 COMPANY SNAPSHOT
15.15.2 REVENUE ANALYSIS
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENTS
15.16 OXFORD PHARMASCIENCE LTD
15.16.1 COMPANY SNAPSHOT
15.16.2 TECHNOLOGY PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 SPECTRUM PHARMACEUTICALS, INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 SUN PHARMACEUTICAL INDUSTRIES LTD.
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENTS
15.19 TERSERA THERAPEUTICS LLC
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
15.2 TEVA PHARMACEUTICALS USA, INC. (A SUBSIDIARY OF TEVA PHARMACEUTICAL INDUSTRIES LTD.)
15.20.1 COMPANY SNAPSHOT
15.20.2 REVENUE ANALYSIS
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
LIST OF TABLES
TABLE 1 NEW CANCER CASES, AGES 85+, IN THE U.S.
TABLE 2 MORTALITY DUE TO CANCER, AGES 85+, IN THE U.S.
TABLE 3 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE 2020-2028 (USD MILLION)
TABLE 4 EUROPE GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 5 EUROPE GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 6 EUROPE ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 7 EUROPE ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 8 EUROPE OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 9 EUROPE OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 10 EUROPE MONOCLONAL ANTIBODIES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 11 EUROPE NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 12 EUROPE NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 13 EUROPE OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 14 EUROPE PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 15 EUROPE BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 16 EUROPE BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 17 EUROPE ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 18 EUROPE ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 19 EUROPE ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 20 EUROPE ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 21 EUROPE OTHERS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 22 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE 2020-2028 (USD MILLION)
TABLE 23 EUROPE BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 24 EUROPE BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 25 EUROPE GENERICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 26 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2020-2028 (USD MILLION)
TABLE 27 EUROPE LUNG CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 28 EUROPE BREAST CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 29 EUROPE PROSTATE CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 30 EUROPE LIVER CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 31 EUROPE BLADDER CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 32 EUROPE LEUKAEMIA IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 33 EUROPE MELANOMA IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 34 EUROPE OVARIAN CANCER IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 35 EUROPE OTHER CANCERS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 36 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2020-2028 (USD MILLION)
TABLE 37 EUROPE HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 38 EUROPE HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 39 EUROPE CLINICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 40 EUROPE HOSPITALS & ACADEMIC INSTITUTIONS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 41 EUROPE OTHERS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 42 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2020-2028 (USD MILLION)
TABLE 43 EUROPE HOSPITAL PHARMACIES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 44 EUROPE RETAIL PHARMACIES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 45 EUROPE COMPOUNDING PHARMACIES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 46 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY COUNTRY 2019-2028 (USD MILLION)
TABLE 47 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 48 EUROPE GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 49 EUROPE OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 50 EUROPE ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 51 EUROPE NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 52 EUROPE OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 53 EUROPE PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 54 EUROPE BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 55 EUROPE ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 56 EUROPE ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 57 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 58 EUROPE BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 59 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 60 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 61 EUROPE HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 62 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 63 GERMANY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 64 GERMANY GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 65 GERMANY OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 66 GERMANY ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 67 GERMANY NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 68 GERMANY OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 69 GERMANY PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 70 GERMANY BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 71 GERMANY ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 72 GERMANY ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 73 GERMANY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 74 GERMANY BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 75 GERMANY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 76 GERMANY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 77 GERMANY HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 78 GERMANY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 79 FRANCE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 80 FRANCE GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 81 FRANCE OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 82 FRANCE ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 83 FRANCE NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 84 FRANCE OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 85 FRANCE PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 86 FRANCE BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 87 FRANCE ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 88 FRANCE ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 89 FRANCE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 90 FRANCE BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 91 FRANCE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 92 FRANCE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 93 FRANCE HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 94 FRANCE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 95 U.K CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 96 U.K GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 97 U.K OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 98 U.K ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 99 U.K NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 100 U.K OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 101 U.K PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 102 U.K BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 103 U.K ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 104 U.K ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 105 U.K CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 106 U.K BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 107 U.K CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 108 U.K CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 109 U.K HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 110 U.K CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 111 ITALY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 112 ITALY GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 113 ITALY OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 114 ITALY ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 115 ITALY NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 116 ITALY OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 117 ITALY PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 118 ITALY BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 119 ITALY ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 120 ITALY ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 121 ITALY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 122 ITALY BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 123 ITALY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 124 ITALY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 125 ITALY HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 126 ITALY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 127 SPAIN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 128 SPAIN GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 129 SPAIN OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 130 SPAIN ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 131 SPAIN NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 132 SPAIN OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 133 SPAIN PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 134 SPAIN BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 135 SPAIN ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 136 SPAIN ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 137 SPAIN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 138 SPAIN BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 139 SPAIN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 140 SPAIN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 141 SPAIN HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 142 SPAIN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 143 SWITZERLAND CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE 2019-2028 (USD MILLION)
TABLE 144 SWITZERLAND GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 145 SWITZERLAND OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 146 SWITZERLAND ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 147 SWITZERLAND NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 148 SWITZERLAND OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION) (USD MILLION)
TABLE 149 SWITZERLAND PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 150 SWITZERLAND BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 151 SWITZERLAND ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 152 SWITZERLAND ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 153 SWITZERLAND CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 154 SWITZERLAND BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 155 SWITZERLAND CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 156 SWITZERLAND CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 157 SWITZERLAND HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 158 SWITZERLAND CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 159 NETHERLANDS CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE 2019-2028 (USD MILLION)
TABLE 160 NETHERLANDS GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 161 NETHERLANDS OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 162 NETHERLANDS ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 163 NETHERLANDS NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 164 NETHERLANDS OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION) (USD MILLION)
TABLE 165 NETHERLANDS PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 166 NETHERLANDS BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 167 NETHERLANDS ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 168 NETHERLANDS ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 169 NETHERLANDS CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 170 NETHERLANDS BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 171 NETHERLANDS CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 172 NETHERLANDS CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 173 NETHERLANDS HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 174 NETHERLANDS CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 175 RUSSIA CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 176 RUSSIA GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 177 RUSSIA OPOID ANALGESIC IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 178 RUSSIA ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 179 RUSSIA NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 180 RUSSIA OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 181 RUSSIA PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 182 RUSSIA BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 183 RUSSIA ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 184 RUSSIA ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 185 RUSSIA CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 186 RUSSIA BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 187 RUSSIA CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 188 RUSSIA CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 189 RUSSIA HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 190 RUSSIA CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 191 TURKEY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 192 TURKEY GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 193 TURKEY OPIOID ANALGESICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 194 TURKEY ERYTHROPOIETIN STIMULATING AGENTS (ESA’S) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 195 TURKEY NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 196 TURKEY OTC NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 197 TURKEY PRESCRIPTION NSAIDS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 198 TURKEY BISPHOSPHONATES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 199 TURKEY ANTI-EMETICS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 200 TURKEY ANTIHISTAMINES IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
TABLE 201 TURKEY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 202 TURKEY BRANDED IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 203 TURKEY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY CANCER TYPE, 2019-2028 (USD MILLION)
TABLE 204 TURKEY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 205 TURKEY HOSPITALS IN CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 206 TURKEY CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 207 REST OF EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET, BY DRUG TYPE, 2019-2028 (USD MILLION)
List of Figure
LIST OF FIGURES
FIGURE 1 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: SEGMENTATION
FIGURE 2 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: SEGMENTATION
FIGURE 10 GROWING CANCER BUREN WORLDWIDE IS EXPECTED TO DRIVE THE EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 11 GRANULOCYTE COLONY STIMULATING FACTOR (GCSFS) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET IN 2021 & 2028
FIGURE 12 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET
FIGURE 13 ESTIMATED NUMBER OF NEW CASES IN 2018, WORLDWIDE, BOTH SEXES, ALL AGES
FIGURE 14 ESTIMATED AGE-STANDARDIZED INCIDENCE RATES (WORLD) IN 2018, WORLDWIDE, BOTH SEXES, ALL AGES
FIGURE 15 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DRUG TYPE, 2020
FIGURE 16 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DRUG TYPE, 2020-2028 (USD MILLION)
FIGURE 17 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DRUG TYPE, CAGR (2021-2028)
FIGURE 18 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 19 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY TYPE, 2020
FIGURE 20 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY TYPE 2020-2028 (USD MILLION)
FIGURE 21 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 22 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 23 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY CANCER TYPE, 2020
FIGURE 24 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY CANCER TYPE, 2020-2028 (USD MILLION)
FIGURE 25 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY CANCER TYPE, CAGR (2021-2028)
FIGURE 26 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 27 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY END USER, 2020
FIGURE 28 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY END USER, 2020-2028 (USD MILLION)
FIGURE 29 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY END USER, CAGR (2021-2028)
FIGURE 30 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 32 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, 2020-2028 (USD MILLION)
FIGURE 33 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 34 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 35 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: SNAPSHOT (2020)
FIGURE 36 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY COUNTRY (2020)
FIGURE 37 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY COUNTRY (2021 & 2028)
FIGURE 38 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY COUNTRY (2021 & 2028)
FIGURE 39 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: BY TYPE (2021-2028)
FIGURE 40 EUROPE CANCER SUPPORTIVE CARE PRODUCTS MARKET: COMPANY SHARE 2020 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.