Market Analysis and Insights: Europe Clinical Trial Supplies Market
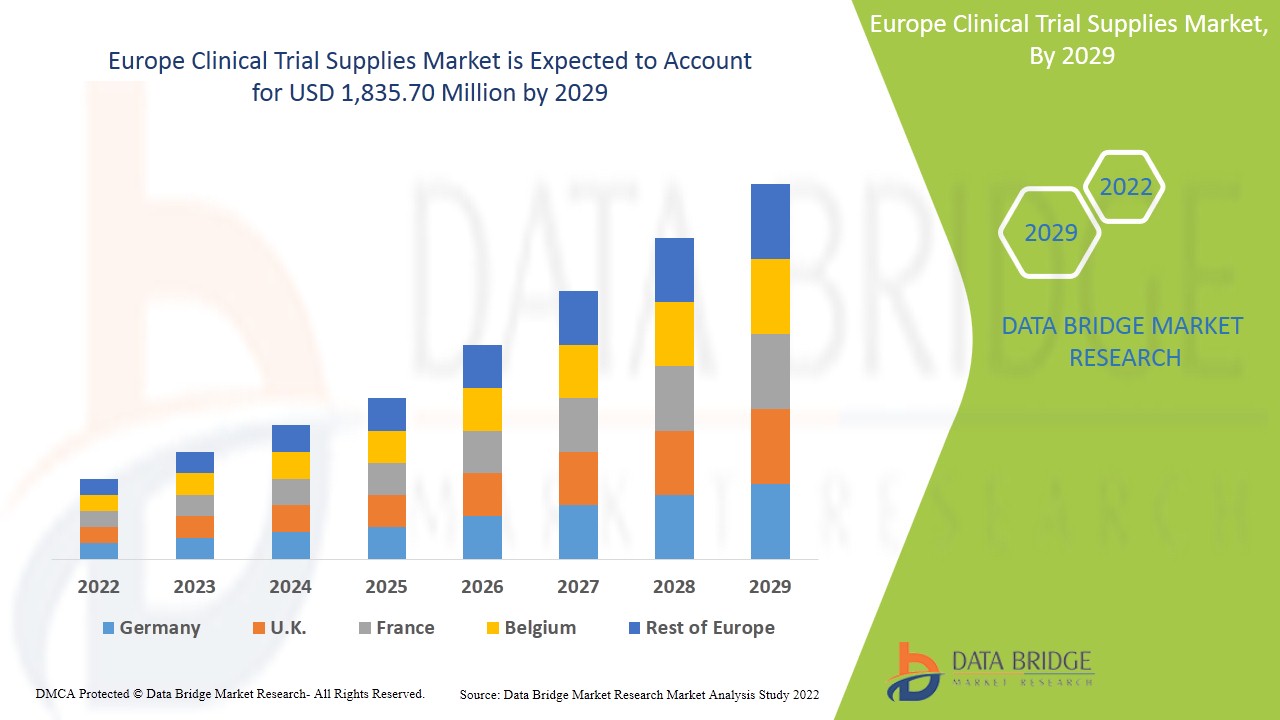
Europe clinical trial supplies market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 7.8% in the forecast period of 2022 to 2029 and is expected to reach USD 1,835.70 million by 2029. The major factor driving the growth of the clinical trial supplies market are rising of demand in clinical trial worldwide, increasing incidences of diseases, government funds to R&D investments and development of new treatments such as personalized medicine leading the clinical trial supplies market to grow in future.
The clinical trial is a research study that determines whether a medical strategy, treatment, or device is safe, effective and useful for humans use. These studies help in find which medical approaches experiment is best for certain diseases. A clinical trial provides the best data for health care decision-making purposes.
The purpose of clinical trial is to study strict scientific standards. These standards protect patients and help in producing reliable study results.
Clinical trial are last stage in drug development in a long and careful research process that is carried out by scientists or researchers for a particular disease, whether drug or medical device. The process drug development often begins in a lab, where scientists first develop and test new ideas related to treatment of disease.
The Europe clinical trial supplies market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, products approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
By Services (Storage, Manufacturing, Packaging And Labelling), Clinical Phase (Phase III, Phase II, Phase IV, Phase I), Therapeutic Uses (Oncology, Cardiovascular Diseases, Dermatology, Metabolic Disorders, Infectious Diseases, Respiratory Diseases, CNS And Mental Disorders, Blood Disorders, Others), By End User (Contract Research Organizations, Pharmaceutical And Biotechnology Companies) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
Movianto (U.S.), Sharp (U.S.),Thermo Fisher Scientific Inc.,(U.S.), Catalent, Inc (U.S.), PCI Pharma Services (U.S.), Almac Group (U.K.), PAREXEL International Corporation (U.S.), Bionical Ltd. (U.K.), Alium Medical Limited (U.K.), Myonex (U.K.), Clinigen Group plc (U.K.), Ancillare, LP (U.S.), SIRO Clinpharm (India) CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (U.S.) Biocair (U.K.) and among others. |
Clinical Trial Supplies Market Dynamics
Drivers
- Rising Demand for Clinical Trial Worldwide
The rising demand for clinical trial has been 82% alone in developing countries such as North America, Europe, and Asia. These drugs are available in the market after clinical trial, so all the companies mostly carry out clinical trial depending on the type of drug or device machine. and hence act as a major driver that will result in the expansion of the growth rate of the treatment market.
- Increasing Incidence of Chronic Diseases
The high prevalence of chronic diseases due to the rapidly increasing population and infections among people can be seen globally. These diseases contribute a major role in the clinical trial field for the development of drugs. The drug has to pass all the standard clinical phase to be available before human consumption. Thus, to treat these chronic diseases for humans, the drug has to be safe.
- Government Funds in R&D Investments
The instruments, workforce, medical management in case of harm to researchers, insurance, transportation, ethics committee fee, data processing, and other consumables lead to major cost involvement in clinical trial. Clinical trial are the evaluation of diseases prevention and treatment ideas will further enhance the growth of treatment market.
Opportunities
- Increasing New Drug Development Trial in Emerging Countries
Clinical trial for drug efficacy is the primary key for drugs development for diseases treatment before launching in the market for human consumption. Additionally, the new drugs have to meet license extensions and international standards before selling and distribution. Increasing the prevalence and incidence of diseases and the rise in the patient’s numbers are the factors leading to emerging trends of clinical trial for drug development in developing countries over the past period.
Also, governments in emerging markets (China, Brazil, Russia, India, and South Africa) reform public healthcare and grant more accessible access to medicine. These two factors working in unison mean greater freedom for market developments and increased innovation in clinical research in emerging markets.
Restraints/Challenges
Adverse drug reactions are the unwanted or harmful effects that can be experienced after the administration of a drug under normal conditions of use in humans. The drug reactions generally occur in jaundice, anaemia, rashes and lead to a decrease in the white blood cell count, damaged kidney, and nerve injury that caused impaired vision or hearing.
Many of the adverse effects may be ascertained from physical examinations during the clinical phase of testing. Thus, reporting adverse effects during clinical trial is the major leading restraints factor for the supplies market. Despite high time and cost investments for developing biologics and new drugs, it is estimated that lower procedure time and rate for approval of drug is creating a biggest challenge for the market, which may hamper the market growth.
This clinical trial supplies market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on clinical trial supplies market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In February 2022, Thermo Fisher Scientific has announced partnership with Medidata to optimize clinical research site selection and speed patient enrollment in clinical trial. This improves clinical trial planning and execution to accelerate clinical trial in which datasets have been generated from 26,000 clinical trial and nearly 8 million patients in more than 140 countries across the globe
Europe Clinical Trial Supplies Market Scope
Europe clinical trial supplies market is categorized based on services, clinical phase, therapeutic uses and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service
- Manufacturing
- Distribution
- Storage
- Packaging and Labelling
On the basis of services the Europe clinical trial supplies market is segmented into manufacturing, distribution, storage, and packaging and labelling.
Clinical Phase
- Phase I
- Phase II
- Phase III
- Phase IV
On the basis of clinical phase the Europe clinical trial supplies market is segmented into phase I, phase II, phase III and phase IV.
Therapeutic Uses
- Oncology
- CNS
- Mental Disorders
- Cardiovascular Diseases
- Infectious Diseases
- Respiratory Diseases
- Blood Disorder
- Dermatology
- Others
On the basis of therapeutic uses, the Europe clinical trial supplies market is segmented into oncology, CNS and mental disorders, cardiovascular diseases, infectious disease, respiratory diseases, metabolic disorders, blood disorders, dermatology and others.
End User
- Contract research organizations
- Pharmaceutical and biotechnology companies
On the basis of end user, the Europe clinical trial supplies market is segmented into contract research organizations and pharmaceutical and biotechnology companies.
Clinical Trial Supplies Market Regional Analysis/Insights
The Europe clinical trial supplies market is further segmented into major countries such as Germany, France, U.K., Italy, Spain, Russia, Belgium, Turkey, Netherlands, Switzerland, rest of Europe.
U.K. dominates the Europe clinical trial supplies market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period of 2022-2029. This is due to the presence of major key players investing in R&D for the better service of clinical trial supplies and well-developed healthcare infrastructure in this region.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Clinical Trial Supplies Market Share Analysis
Europe clinical trial supplies market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trial pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies’ focus related to clinical trial supplies market.
The major prominent participants operating in the Europe clinical trial supplies market are Movianto (U.S.), Sharp (U.S.),Thermo Fisher Scientific Inc.,(U.S.), Catalent, Inc (U.S.), PCI Pharma Services (U.S.), Almac Group (U.K.), PAREXEL International Corporation (U.S.), Bionical Ltd. (U.K.), Alium Medical Limited (U.K.), MYODERM (U.K.), Clinigen Group plc (U.K.), Ancillare, LP (U.S.), SIRO Clinpharm (India) CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (U.S.) Biocair (U.K.) and among others.
Research Methodology: Europe Clinical Trial Supplies Market
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. In adition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, GlobalVsRegional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CLINICAL TRIAL SUPPLIES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES
5 EUROPE CLINICAL TRIAL SUPPLIES MARKET: REGULATORY SCENARIO
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING DEMAND FOR CLINICAL TRIALS WORLDWIDE
6.1.2 INCREASING INCIDENCE OF CHRONIC DISEASES
6.1.3 GOVERNMENT FUNDS IN R&D INVESTMENTS
6.1.4 ADVANCEMENT OF TECHNOLOGY IN CLINICAL TRIALS SUPPLIES
6.2 RESTRAINTS
6.2.1 ADVERSE EFFECTS OF CLINICAL TRIALS
6.2.2 TRANSPORTATION ISSUE IN CLINICAL TRIAL SUPPLIES
6.2.3 HIGH COST ASSOCIATED WITH THE CLINICAL TRIALS
6.3 OPPORTUNITIES
6.3.1 INCREASING NEW DRUG DEVELOPMENT TRIALS IN EMERGING COUNTRIES
6.3.2 INCREASING DEMAND FOR INNOVATIVE SOLUTIONS IN CLINICAL TRIALS SERVICES
6.3.3 EVOLUTION IN SUPPLY CHAIN MANAGEMENT FOR CLINICAL TRIALS
6.4 CHALLENGES
6.4.1 LOWER PROCEDURE TIME OF CLINICAL TRIALS APPROVAL
6.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CLINICAL TRIALS
7 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES
7.1 OVERVIEW
7.2 STORAGE
7.3 MANUFACTURING
7.4 PACKAGING AND LABELLING
7.5 DISTRIBUTION
8 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASES
8.1 OVERVIEW
8.2 PHASE III
8.3 PHASE II
8.4 PHASE IV
8.5 PHASE I
9 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE
9.1 OVERVIEW
9.2 ONCOLOGY
9.3 CARDIOVASCULAR DISEASES
9.4 DERMATOLOGY
9.5 METABOLIC DISORDERS
9.6 INFECTIOUS DISEASES
9.7 RESPIRATORY DISEASES
9.8 CNS AND MENTAL DISORDERS
9.9 BLOOD DISORDERS
9.1 OTHERS
10 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY END USER
10.1 OVERVIEW
10.2 CONTRACT RESEARCH ORGANIZATIONS
10.3 PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES
11 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY REGION
11.1 EUROPE
11.1.1 GERMANY
11.1.2 FRANCE
11.1.3 U.K.
11.1.4 ITALY
11.1.5 SPAIN
11.1.6 NETHERLANDS
11.1.7 RUSSIA
11.1.8 BELGIUM
11.1.9 TURKEY
11.1.10 SWITZERLAND
11.1.11 REST OF EUROPE
12 EUROPE CLINICAL TRIAL SUPPLIES MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: EUROPE
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 THERMO FISHER SCIENTIFIC INC.
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENT
14.1.5.1 PARTNERSHIP
14.2 ALMAC GROUP
14.2.1 COMPANY SNAPSHOT
14.2.2 COMPANY SHARE ANALYSIS
14.2.3 PRODUCT PORTFOLIO
14.2.4 RECENT DEVELOPMENTS
14.3 CATALENT INC.
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 SERVICE PORTFOLIO
14.3.5 RECENT DEVELOPMENT
14.3.5.1 SERVICE EXPANSION
14.4 CLINIGEN GROUP PLC
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENT
14.4.5.1 PARTNERSHIP
14.5 MOVIANTO
14.5.1 COMPANY SNAPSHOT
14.5.2 COMPANY SHARE ANALYSIS
14.5.3 SERVICE PORTFOLIO
14.5.4 RECENT DEVELOPMENT
14.5.4.1 ACQUISITION
14.6 PCI PHARMA SERVICES
14.6.1 COMPANY SNAPSHOT
14.6.2 SERVICE PORTFOLIO
14.6.3 RECENT DEVELOPMENTS
14.7 SHARP
14.7.1 COMPANY SNAPSHOT
14.7.2 SERVICE PORTFOLIO
14.7.3 RECENT DEVELOPMENT
14.8 ALIUM MEDICAL LIMITED
14.8.1 COMPANY SNAPSHOT
14.8.2 SERVICE PORTFOLIO
14.8.3 RECENT DEVELOPMENT
14.9 ANCILLARE, LP
14.9.1 COMPANY SNAPSHOT
14.9.2 SERVICE PORTFOLIO
14.9.3 RECENT DEVELOPMENT
14.1 BIOCAIR
14.10.1 COMPANY SNAPSHOT
14.10.2 SERVICE PORTFOLIO
14.10.3 RECENT DEVELOPMENTS
14.11 BIONICAL LTD.
14.11.1 COMPANY SNAPSHOT
14.11.2 SERVICE PORTFOLIO
14.11.3 RECENT DEVELOPMENT
14.11.3.1 SERVICE LAUNCH
14.12 CLINICAL SUPPLIES MANAGEMENT HOLDINGS,INC
14.12.1 COMPANY SNAPSHOT
14.12.2 SERVICE PORTFOLIO
14.12.3 RECENT DEVELOPMENT
14.13 KLIFO
14.13.1 COMPANY SNAPSHOT
14.13.2 SERVICE PORTFOLIO
14.13.3 RECENT DEVELOPMENTS
14.13.3.1 ACQUISTION
14.14 MYONEX
14.14.1 COMPANY SNAPSHOT
14.14.2 SERVICE PORTFOLIO
14.14.3 RECENT DEVELOPMENT
14.15 PAREXEL INTERNATIONAL CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 SERVICE PORTFOLIO
14.15.3 RECENT DEVELOPMENT
14.15.3.1 COLLABORATION
14.16 SIRO CLINPHARM PRIVATE LIMITED
14.16.1 COMPANY SNAPSHOT
14.16.2 SERVICE PORTFOLIO
14.16.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
List of Table
TABLE 1 LOCATIONS OF REGISTERED STUDIES
TABLE 2 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 3 EUROPE STORAGE IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE MANUFACTURING IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE PACKAGING AND LABELLING IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE DISTRIBUTION IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE PHASE III IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE PHASE II IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE PHASE IV IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE PHASE I IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 13 EUROPE ONCOLOGY IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE CARDIOVASCULAR DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE DERMATOLOGY IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE METABOLIC DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE INFECTIOUS DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE RESPIRATORY DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE CNS AND MENTAL DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE BLOOD DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE OTHERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 23 EUROPE CONTRACT RESEARCH ORGANIZATIONS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 26 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 27 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 29 EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 30 GERMANY CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 31 GERMANY CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 32 GERMANY CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 33 GERMANY CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 FRANCE CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 35 FRANCE CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 36 FRANCE CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 37 FRANCE CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 38 U.K. CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 U.K. CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 40 U.K. CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 41 U.K. CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 ITALY CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 43 ITALY CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 44 ITALY CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 45 ITALY CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 SPAIN CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 SPAIN CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 48 SPAIN CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 49 SPAIN CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 NETHERLANDS CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 51 NETHERLANDS CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 52 NETHERLANDS CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 53 NETHERLANDS CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 RUSSIA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 55 RUSSIA CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 56 RUSSIA CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 57 RUSSIA CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 58 BELGIUM CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 BELGIUM CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 60 BELGIUM CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 61 BELGIUM CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 62 TURKEY CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 63 TURKEY CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 64 TURKEY CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 65 TURKEY CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 66 SWITZERLAND CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 SWITZERLAND CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 68 SWITZERLAND CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 69 SWITZERLAND CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 70 REST OF EUROPE CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 EUROPE CLINICAL TRIAL SUPLLIES MARKET: SEGMENTATION
FIGURE 2 EUROPE CLINICAL TRIAL SUPPLIES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CLINICAL TRIAL SUPPLIES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CLINICAL TRIAL SUPPLIES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE CLINICAL TRIAL SUPPLIES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CLINICAL TRIAL SUPLLIES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CLINICAL TRIAL SUPPLIES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CLINICAL TRIAL SUPPLIES MARKET: DBMR VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE CLINICAL TRIAL SUPPLIES MARKET: SEGMENTATION
FIGURE 10 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE CLINICAL TRIAL SUPPLIES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 11 RISING DEMAND FOR CLINICAL TRIALS WORLDWIDE AND INCREASING INCIDENCES OF DISEASES IS DRIVING THE EUROPE CLINICAL TRIAL SUPPLIES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 STORAGE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CLINICAL TRIAL SUPPLIES MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE CLINICAL TRIAL SUPPLIES MARKET
FIGURE 14 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, 2021
FIGURE 15 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 16 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 17 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 18 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, 2021
FIGURE 19 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, 2022-2029 (USD MILLION)
FIGURE 20 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, CAGR (2022-2029)
FIGURE 21 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, LIFELINE CURVE
FIGURE 22 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, 2021
FIGURE 23 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, 2022-2029 (USD MILLION)
FIGURE 24 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, CAGR (2022-2029)
FIGURE 25 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, LIFELINE CURVE
FIGURE 26 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY END USER, 2021
FIGURE 27 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 28 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 29 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 EUROPE CLINICAL TRIAL SUPPLIES MARKET: SNAPSHOT (2021)
FIGURE 31 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2021)
FIGURE 32 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 33 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 34 EUROPE CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES (2022-2029)
FIGURE 35 EUROPE CLINICAL TRIAL SUPPLIES MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.