Europe Craniomaxilofacial Devices Maket
Market Size in USD Million
CAGR :
% 
 USD
197.80 Million
USD
295.83 Million
2024
2032
USD
197.80 Million
USD
295.83 Million
2024
2032
| 2025 –2032 | |
| USD 197.80 Million | |
| USD 295.83 Million | |
|
|
|
|
Craniomaxillofacial Devices Market Size
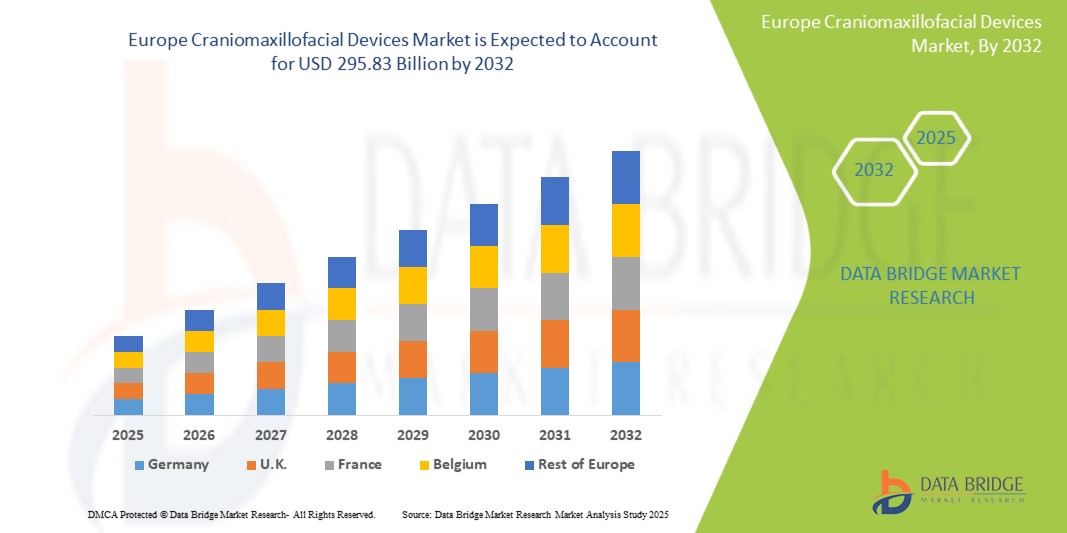
- The Europe Craniomaxillofacial Devices Market was valued at USD 197.8 Million in 2024 and is expected to reach USD 295.83 Million by 2032, at a CAGR of 6.5% during the forecast period.
- The growth of the Europe Craniomaxillofacial Devices Market is driven by several key factors. One of the primary drivers is the increasing incidence of traumatic injuries and facial fractures, often resulting from accidents, sports injuries, and violent incidents. These injuries require advanced surgical interventions and the use of craniomaxillofacial devices for effective treatment and rehabilitation. Additionally, the rising demand for aesthetic procedures, including facial reconstructive surgeries, has spurred the market for devices that aid in the restoration of facial appearance and functionality.
Europe Craniomaxillofacial Devices Market Analysis
- Craniomaxillofacial devices are vital in the treatment of facial trauma, congenital deformities, and reconstructive surgeries, playing an essential role in restoring both functionality and aesthetic appearance. These devices are used in a variety of medical settings, including hospitals, specialty clinics, and rehabilitation centers, and are crucial for patients undergoing surgeries related to facial bone fractures, tumors, or deformities.
- The demand for craniomaxillofacial devices in Europe is primarily driven by the increasing incidence of facial injuries and fractures due to accidents, sports injuries, and violence. Additionally, the aging population, which often faces conditions such as bone loss or fractures, contributes to the rising need for reconstructive procedures. The growing focus on facial aesthetic surgeries and cosmetic reconstructive procedures is further propelling the demand for these devices across the region.
- Europe holds a substantial share of the global craniomaxillofacial devices market, owing to its advanced healthcare infrastructure, cutting-edge medical research, and highly skilled healthcare professionals. Countries such as Germany, France, and the United Kingdom are at the forefront due to their well-established healthcare systems, strong emphasis on trauma care, and advanced surgical techniques. These countries also benefit from favorable reimbursement policies and increasing patient awareness of available treatment options.
- The European market is also bolstered by regulatory standards set by the European Medicines Agency (EMA) and the Medical Device Regulation (MDR), which ensure the safety and efficacy of these devices. Moreover, technological advancements such as 3D printing, custom implants, and minimally invasive surgical techniques are enhancing the precision and success rates of craniomaxillofacial procedures, making them more accessible and effective.
- Additionally, the growing popularity of aesthetic procedures, including facial reconstructive surgeries for congenital and traumatic deformities, is contributing to the market's growth. As the demand for both functional and cosmetic solutions increases, alongside the rising number of elderly patients requiring facial surgeries, the craniomaxillofacial devices market in Europe is expected to experience steady expansion in the coming years.
Report Scope Craniomaxillofacial Devices Market Segmentation
|
Attributes |
Craniomaxillofacial Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Craniomaxillofacial Devices Market Trends
“Personalized Implants and Technological Advancements in Surgical Precision”
- Advancements in 3D printing are transforming the craniomaxillofacial devices market by enabling the production of patient-specific implants, enhancing surgical accuracy, reducing operative time, and improving aesthetic and functional outcomes.
- The integration of computer-assisted surgical planning and navigation systems is gaining popularity, allowing for greater precision in complex craniofacial reconstructions and trauma procedures.
- For instance, Germany's highly developed healthcare system, along with its emphasis on trauma care and facial reconstruction, has resulted in a steady demand for craniomaxillofacial devices, particularly for maxillofacial surgeries and post-trauma treatments.
- Rising demand for minimally invasive procedures is driving the adoption of smaller, anatomically contoured implants that reduce scarring, accelerate recovery, and improve patient satisfaction.
- The growing use of biocompatible and resorbable materials is creating new opportunities for long-term safety and reduced need for secondary surgeries, particularly in pediatric and trauma patients.
Craniomaxillofacial Devices Market Dynamics
Driver
“Rising Incidence of Facial Trauma and Growing Demand for Advanced Reconstructive Solutions”
- The Europe craniomaxillofacial devices market is primarily driven by the increasing number of facial injuries resulting from road accidents, sports activities, and physical trauma, which necessitate surgical intervention and the use of specialized implants.
- Aging demographics across countries like Germany, France, and Italy contribute to a higher prevalence of age-related craniofacial conditions and fractures, further increasing the need for reconstructive and corrective procedures.
- There is a growing emphasis on aesthetic and functional reconstruction, with rising demand for facial surgeries due to congenital anomalies, tumor resections, and cosmetic enhancements, propelling the adoption of craniomaxillofacial devices.
- Technological innovations, including 3D printing, virtual surgical planning (VSP), and patient-specific implants, are enhancing surgical precision, reducing operative time, and improving post-surgical outcomes.
For instance,
- In 2023Medartis AG (Switzerland) introduced a new line of titanium 3D-printed craniofacial implants tailored to individual patient anatomies, significantly improving fit and reducing post-operative complications
- This growth is further supported by regulatory advancements under the EU Medical Device Regulation (MDR), increasing surgical expertise across Europe, and the expansion of trauma and specialty care units, making advanced craniomaxillofacial treatments more widely available.
Opportunity
“Advancement of Personalized Craniomaxillofacial Solutions for Outpatient and Ambulatory Surgical Settings”
- The shift toward outpatient and ambulatory care across Europe is creating new opportunities for craniomaxillofacial devices that support faster recovery, minimal hospitalization, and reduced surgical complications through minimally invasive approaches and tailored implants.
- Increasing demand for same-day surgeries and enhanced recovery protocols is driving the adoption of patient-specific implants and digitally planned procedures, enabling high precision and reduced operative time in decentralized clinical environments.
- The integration of virtual surgical planning (VSP), 3D printing, and augmented reality in outpatient settings is allowing surgeons to pre-plan complex craniofacial procedures with greater accuracy and deliver optimal outcomes outside traditional hospital systems.
For instance,
- In February 2024, KLS Martin Group launched a virtual planning and 3D-printed implant platform designed specifically for outpatient craniomaxillofacial surgeries, enabling personalized care and streamlining workflows for surgeons across decentralized surgical centers in Europe
- This opportunity is further enhanced by Europe's rising healthcare digitization, growing demand for customized care, and increasing pressure to reduce hospital stays and healthcare expenditures—driving the need for efficient, tech-enabled craniomaxillofacial treatment solutions.
Restraint/Challenge
“High Costs and Stringent EU MDR Compliance Hindering Market Accessibility and Innovation”
- The high cost of craniomaxillofacial devices—particularly patient-specific implants, bioresorbable materials, and computer-assisted surgical systems—poses a significant barrier to adoption in budget-constrained healthcare systems and among underserved patient populations across Europe.
- Strict regulatory requirements under the EU Medical Device Regulation (MDR) mandate comprehensive clinical data, ongoing surveillance, and detailed documentation, increasing the time and cost required to bring new craniomaxillofacial products to market.
- Small and medium-sized enterprises (SMEs) often face resource limitations that make MDR compliance especially burdensome, leading to delays, reduced innovation, or market withdrawal—affecting device availability and competition in key European regions.
For instance,
- In 2024, a MedTech Europe analysis revealed that nearly 60% of SMEs in the craniomaxillofacial segment reported delayed product launches or scaled-back R&D investments due to regulatory and financial hurdles linked to MDR adaptation.
- These challenges are further compounded in rural and decentralized healthcare settings, where limited budgets and logistical barriers can restrict access to advanced surgical solutions—ultimately constraining market expansion and patient outcomes across Europe.
Craniomaxillofacial Devices Market Scope
The market is segmented on the basis, product, type, application, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
By Material |
|
|
By Location |
|
|
By Application |
|
|
By End User |
|
In 2025, the Ankle Braces and Supports Segment is Projected to Dominate the Market with the Largest Share in the Product Segment
The ankle braces and supports segment is anticipated to lead the Europe Orthopaedic Braces and Supports Market with the largest share of 34.14% in 2025, due to its high rising prevalence of ankle sprains, ligament injuries, and fractures, particularly among athletes and individuals with active lifestyles. These devices are essential for providing joint stability, minimizing swelling, and facilitating safe mobility during recovery. The increasing focus on injury prevention in sports and physical fitness, along with growing awareness of early intervention and rehabilitation, is fueling demand for ankle supports. Additionally, advancements in product design—such as lightweight, breathable, and anatomically contoured materials—are enhancing patient comfort and compliance. The availability of adjustable and customizable options is also expanding the user base across different age groups and activity levels. Moreover, the integration of smart technologies, such as embedded sensors for real-time motion and pressure monitoring, is transforming ankle braces into advanced rehabilitation tools. With the expanding emphasis on non-invasive treatments, home-based recovery, and personalized orthopaedic care, the ankle braces and supports segment is expected to maintain a leading position in the European market.
Neurosurgery and ENT Clinics are Expected to Account for the Largest Share During the Forecast Period in plastic surgery Market
In 2025, Neurosurgery and ENT (Ear, Nose, and Throat) clinics are projected to hold the largest share of 38.31% driven by the increasing demand for craniomaxillofacial surgeries related to neurological and ENT conditions. These clinics play a crucial role in managing complex craniofacial trauma, deformities, and conditions such as facial nerve disorders, skull fractures, and tumors. Neurosurgery and ENT clinics across Europe, particularly in advanced healthcare systems in Germany, France, and the U.K., are investing in cutting-edge craniomaxillofacial devices to enhance surgical precision, recovery outcomes, and patient satisfaction. The adoption of minimally invasive surgical tools, 3D-printed implants, and advanced fixation devices is improving the efficiency of procedures and reducing recovery times. Additionally, the integration of personalized care solutions and the growing emphasis on outpatient and post-operative rehabilitation further strengthens the dominance of these clinics as a key distribution channel for craniomaxillofacial devices.
Craniomaxillofacial Devices Market Regional Analysis
“Germany is the Dominant Country in the Craniomaxillofacial Devices Market”
- Germany leads the Europe Craniomaxillofacial Devices Market, holding the largest share due to its advanced healthcare infrastructure, high demand for trauma and reconstructive surgeries, and substantial investments in medical technologies.
- The country’s strong emphasis on maxillofacial trauma care, facial deformities, and reconstructive surgery drives the adoption of craniomaxillofacial devices across hospitals, specialized trauma centers, and outpatient surgical facilities.
- Germany's well-established healthcare system, coupled with favorable reimbursement policies, provides a conducive environment for the growth of craniomaxillofacial device adoption, particularly in both aesthetic and functional reconstructive procedures.
- The presence of leading manufacturers such as Medartis AG and KLS Martin Group, along with the country's strong research and development ecosystem, further cements Germany’s market leadership.
- The integration of advanced technologies such as 3D printing, virtual surgical planning, and patient-specific implants is also strengthening Germany’s position as a hub for craniomaxillofacial innovations in Europe.
“U.K. is Projected to Register the Highest Growth Rate”
- The U.K. is expected to experience the fastest growth in the Europe Craniomaxillofacial Devices Market, driven by an increasing prevalence of facial trauma, congenital defects, and the growing demand for reconstructive and cosmetic surgeries.
- The U.K. government’s focus on improving healthcare access, particularly through the integration of digital health technologies and telemedicine, is accelerating the adoption of advanced craniomaxillofacial devices, including 3D-printed implants and virtual planning systems.
- Investment in expanding specialized trauma care across rural and underserved areas, combined with the country’s aging population and rise in facial injuries, is fueling demand for craniomaxillofacial products.
- The presence of top healthcare providers and academic research institutions collaborating with medical device companies is driving the development and commercialization of cutting-edge craniomaxillofacial technologies, further boosting the market’s growth in the U.K.
Craniomaxillofacial Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Stryker Corporation (U.S.)
- Johnson & Johnson (DePuy Synthes) (U.S.)
- Zimmer Biomet Holdings, Inc. (U.S.)
- Medartis AG (Switzerland)
- KLS Martin Group (Germany)
- B. Braun Melsungen AG (Germany)
- Evonos GmbH & Co. KG (Germany)
Latest Developments in Global Craniomaxillofacial Devices Market
- In March 2024, KLS Martin Group announced the new 5,100 m² production facility in Jacksonville, Florida. It increases production capacity for custom-made craniomaxillofacial implants. The investment was USD 38 million, integrating state-of-the-art additive manufacturing technologies to accommodate rising demand for individualized implants and to create 180 new jobs contributing to the regional economy.
- In February 2024, According to Materialise, the company released its Personalized TMJ Total Arthroplasty System on February 22, 2024. In a clinical study, one year post-surgery, the system, featuring implants, guides, and digital planning, achieved a 100% success rate. Patients have shown significant improvement in pain levels, quality of life, and their ability to eat.
- In December 2023, Stryker Corporation, one of the world’s leading medical technology companies, announced the execution of a binding offer to Menix to acquire SERF SAS, a France-based joint replacement company.
- In November 2022: Zimmer Biomet Inc., a global medical technology leader, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the Persona OsseoTi Keel Tibia for cementless knee replacement.
- In February 2022, Johnson & Johnson Medical Devices Companies announced that DePuy Synthes (a division of Johnson & Johnson) has acquired CrossRoads Extremity Systems, a Tennessee-based foot and ankle company that provides a broad range of procedure-specific, sterile-packed implants and instrumentation systems cleared for lower extremity indications
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.