Europe Dental Membrane And Bone Graft Substitute Market
Market Size in USD Million
CAGR :
% 
 USD
423.96 Million
USD
876.27 Million
2022
2030
USD
423.96 Million
USD
876.27 Million
2022
2030
| 2023 –2030 | |
| USD 423.96 Million | |
| USD 876.27 Million | |
|
|
|
|
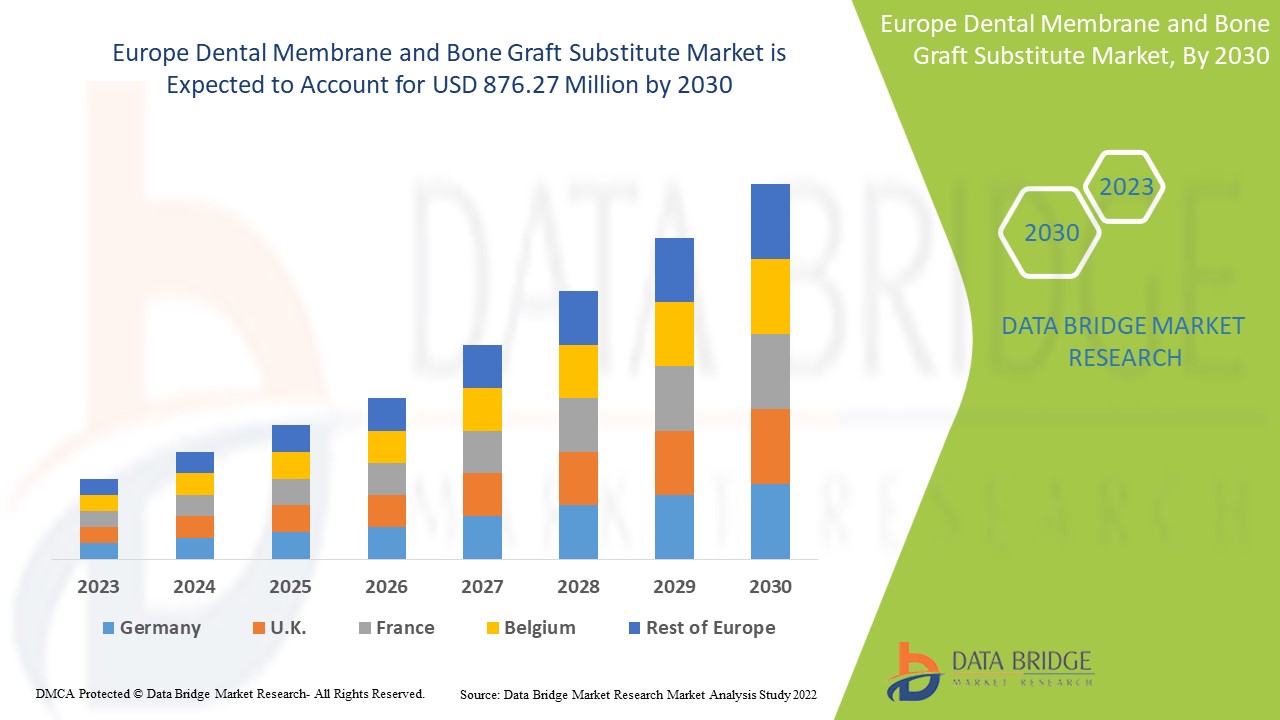
Europe Dental Membrane and Bone Graft Substitute Market Analysis and Size
According to the CDC report, the global incidence rate of hip fracture is expected to rise by 240% in women and 310% in men. It is estimated that 200 million women worldwide suffer from osteoporosis. Bone disorders are common in the elderly population due to low bone density. The number of Americans aged 65 and older is estimated to be around 46 million in 2016, rising to 98 million by 2060. Increasing incidences of musculoskeletal disorders and an ageing population are certain factors that will bring innovation in the dental membrane and bone graft substitute market.
Data Bridge Market Research analyses that the dental membrane and bone graft substitute market which was USD 423.96 million in 2022, is expected to reach USD 876.27 million by 2030, at a CAGR of 9.5% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Dental Membrane and Bone Graft Substitute Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Dental Membranes, and Dental Bone Grafts), Material (Hydrogel, Collagen, Human Cells Source, Hydroxyapatite, Tricalcium Phosphate, Polytetrafluroethylene), Application (Ridge Augmentation, Socket Preservation, Periodontal Defect Regeneration, Implant Bone Regeneration, Sinus Lift, Others), End Use (Hospitals, Dental Clinics, Ambulatory Surgical Centers) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
Zimmer Biomet (U.S.), Bioventus (U.S.), Synergy Orthopedics (U.S.), Ito Co., Ltd. (Japan), Boston Scientific Corporation (U.S.), BTL (Canada), Cyberonics, Inc. (U.S.), DJO LLC (U.S.), Medtronic (Ireland), Nevro Corp (U.S.), NeuroMetrix, Inc (U.S.), Abbott (U.S.), Uroplasty Inc (U.S.), Cogentix Medical (U.S.), Aleva Neurotherapeutics (Switzerland), NeuroPace Inc (U.S.), AxioBionics (U.S.), Beurer GmbH (Germany), Omron Healthcare, Inc (Japan), RS Medical (Japan), Stryker (U.S.) |
|
Market Opportunities |
|
Market Definition
Dental membranes and bone graft substitutes are used in dental bone regeneration procedures in patients suffering from periodontal diseases and oral cancer. Dental membranes are used in conjunction with dental bone grafts to promote cell proliferation during regeneration. A bone graft is an implantable material used to promote bone formation, bone healing, osseous reconstruction, and other procedures.
Europe Dental Membrane and Bone Graft Substitute Market Dynamics
Drivers
- Rising incidence of trauma and accident cases
Osteoarthritis is one of the most common articular diseases in people over the age of 65. According to the Centers for Disease Control and Prevention (CDC), an estimated 63 million Americans have arthritis, which is expected to rise to 78 million by 2040. Furthermore, according to World Population Prospects: The 2019 Revision Data, 1 in 11 people in 2019 were over the age of 65. This figure is expected to rise to one in every six people by 2050.
Furthermore, an increasing incidence of trauma and accident cases worldwide is a major factor likely to boost market demand. According to the National Safety Council, approximately 4.5 million people were severely injured in accidents in 2018. Thus, the above-mentioned factors, the dental membrane and bone graft substitute market is expected to witness significant growth over the forecast period.
- High incidence of periodontal disorders
The rising prevalence of oral health disorders such as periodontal disease and oral cancer is expected to drive the growth of the global dental membranes and bone graft substitutes market during the forecast period. For instance, the National Center for Biotechnology Information (NCBI) article Assessment of the Status of National Oral Health Policy in India, published in 2015, states that the prevalence of oral diseases is very high in India, with dental caries being the most common. It reports that the prevalence is 50.0%, 52.5%, 61.4%, 79.2%, and 84.7% in people aged 5, 12, 15, 35-44, and 65-74, respectively.
- Rising number of bone fracture cases
The increasing number of bone fracture cases and the effectiveness of external bone growth stimulators in healing fractures are driving up product demand. According to a study published in the Archives of Osteoporosis, 2020, fragility fractures accounted for 2.7 million in 2017 in the five largest European Union countries plus Sweden (EU6) and are expected to reach 3.3 million by 2030, a 23% increase from 2017. Furthermore, 14% of the Australian population has osteoporosis, according to a study published in the Orthopaedic Trauma Association International in 2020, and 60,000 hip fractures are expected to occur in Australia by 2050. This reflects the growing global fracture burden. As a result, there is an increasing demand for external bone growth stimulators, which will contribute to the growth of the industry.
- Increased product development
Manufacturers' increased product development and focus on meeting demand for efficient therapeutics are expected to drive market growth during the forecast period. For instance, according to the National Oral Health Survey of India, the prevalence of periodontal diseases in people aged 65 to 74 years was approximately 79.9%, while the incidence of oral cancer in India was approximately 12.6 per 100,000 people in 2016-2017.
Opportunities
- Rise in prevalence of orthopedic diseases
According to the Arthritis Foundation, degenerative joint disease disorders such as osteoarthritis will affect nearly 130 million people worldwide by 2050. The increased prevalence and burden of orthopaedic diseases have resulted in increased patient pool undergoing minimally invasive surgeries worldwide. As a result, dental membrane and bone graft substitutes are in high demand. The high prevalence of bone marrow transplants and bone cancers increases the demand for dental membrane and bone graft substitute.
Restraints/Challenges
- Limited medical reimbursement
Limited medical reimbursement for bone stimulation products and high treatment costs associated with BMP and PRP products will obstruct market growth, while side effects associated with BMP-based orthopaedic treatment will further challenge market growth during the forecast period.
This dental membrane and bone graft substitute market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the dental membrane and bone graft substitute market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Dental Membrane and Bone Graft Substitute Market
The COVID-19 had a negative impact on the dental membrane and bone graft substitute market. Companies are facing a setback lockdown, which has caused disruptions in supply chain activities around the world. The worldwide implementation of lockdown restrictions has resulted in a decrease in patient visits. However, the situation is projected to improve as governments around the world begin to ease guidelines for resuming business activities. Furthermore, the demand for dental membrane and bone graft substitute is expected to rise as elective surgeries and procedures resume in the near future.
Recent developments
- In October 2021, Nobel Biocare launched the GalvoSurge Dental Implant Cleaning System, which completely removes biofilm from bacteria-infected titanium implants. It removes biofilm from difficult-to-reach areas of any bacteria-infected titanium implant surface.
- In January 2021, Dentsply Sirona announced the acquisition of Datum Dental, Ltd. and its extensive portfolio of OSSIX biomaterials. Datum Dental, based in Israel, is well-known for its cutting-edge dental regeneration solutions, which include the clinically superior GLYMATRIX unique technology.
Europe Dental Membrane and Bone Graft Substitute Market Scope
The dental membrane and bone graft substitute market is segmented on the basis of product type, material, application and end-use. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Dental Membranes
- Resorbable
- Non-resorbable
- Dental Bone Grafts
- Autograft
- Allograft
- Demineralized bone matrix
- Xenograft
- Synthetic bone graft substitutes
Material
- Hydrogel
- Collagen
- Human Cells Source
- Hydroxyapatite
- Tricalcium Phosphate
- Polytetrafluroethylene
Application
- Ridge Augmentation
- Socket Preservation
- Periodontal Defect Regeneration
- Implant Bone Regeneration
- Sinus Lift
- Others
End Use
- Hospitals
- Dental Clinics
- Ambulatory Surgical Centers
Europe Dental Membrane and Bone Graft Substitute Market Regional Analysis/Insights
The dental membrane and bone graft substitute market is analyzed and market size insights and trends are provided by country, product type, material, application and end-use as referenced above.
The countries covered in the dental membrane and bone graft substitute market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe
Germany dominates the European region for dental membrane and bone graft substitute because Germany is heavily investing in research and development to provide better patient care. Furthermore, the leading manufacturers in Germany are present, implying that it controls the largest share of the European market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The dental membrane and bone graft substitute market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for dental membrane and bone graft substitute market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the dental membrane and bone graft substitute market. The data is available for historic period 2011-2021.
Competitive Landscape and Dental Membrane and Bone Graft Substitute Market Share Analysis
The dental membrane and bone graft substitute market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to dental membrane and bone graft substitute market.
Some of the major players operating in the dental membrane and bone graft substitute market are:
- Zimmer Biomet (U.S.)
- Bioventus (U.S.)
- Synergy Orthopedics (U.S.)
- Ito Co., Ltd. (Japan)
- Boston Scientific Corporation (U.S.)
- BTL (Canada)
- Cyberonics, Inc. (U.S.)
- DJO LLC (U.S.)
- Medtronic (Ireland)
- Nevro Corp (U.S.)
- NeuroMetrix, Inc (U.S.)
- Abbott (U.S.)
- Uroplasty Inc (U.S.)
- Cogentix Medical (U.S.)
- Aleva Neurotherapeutics (Switzerland)
- NeuroPace Inc (U.S.)
- AxioBionics (U.S.)
- Beurer GmbH (Germany)
- Omron Healthcare, Inc (Japan)
- RS Medical (Japan)
- Stryker (U.S.)
Research Methodology: Europe Dental Membrane and Bone Graft Substitute Market
Data collection and base year analysis is done using data collection modules with large sample sizes. The market data is analyzed and estimated using market statistical and coherent models. Also market share analysis and key trend analysis are the major success factors in the market report. To know more please request an analyst call or can drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Factbook) or can assist you in creating presentations from the data sets available in the report.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY TYPE
17.1 OVERVIEW
17.2 DENTAL MEMBRANE
17.2.1 SYNTHETIC
17.2.1.1. METAL AND METAL REINFORCED MEMBRANES
17.2.1.2. BILAYER POLYLACTIC ACID (PLA) MEMBRANE
17.2.1.3. PTFE MEMBRANE
17.2.1.4. OTHERS
17.2.2 NATRUAL
17.2.2.1. COLLAGEN MEMBRANE
17.2.2.2. ALLOGRAFT DENTAL MEMBRANES
17.2.2.3. OTHERS
17.3 DENTAL BONE GRAFT SUBSITUTE
17.3.1 SYNTHETIC BONE GRAFT
17.3.1.1. TRICALCIUM PHOSPHATE CERAMICS
17.3.1.2. HYDROXYAPATITE
17.3.1.3. BIOACTIVE GLASS
17.3.1.4. BIPHASIC CALCIUM PHOSPHATE CERAMICS
17.3.1.5. CALCIUM PHOSPHATE CEMENT
17.3.1.6. CALCIUM-PHOSPHOSILICATE
17.3.1.7. METALS
17.3.1.7.1. MAGNESIUM (MG)
17.3.1.7.2. STRONTIUM (SR)
17.3.1.7.3. ZINC (ZN)
17.3.1.7.4. SILICON (SI)
17.3.1.8. POLYMERS
17.3.1.8.1. BY USABILITY
17.3.1.8.1.1 DEGRADABLE
17.3.1.8.1.2 NON-DEGRADABLE
17.3.1.8.2. BY TYPE
17.3.1.8.2.1 POLYLACTIC ACID
17.3.1.8.2.2 POLYGLYCOLIC ACID
17.3.1.8.2.3 POLYΕ-CAPROLACTONE
17.3.1.8.2.4 OTHERS
17.3.1.9. COMPOSITES
17.3.1.9.1. BY TYPE
17.3.1.9.1.1 NANOCRYSTALLINE HA/SILICON DIOXIDE
17.3.1.9.1.2 Β-TCP/CALCIUM SULPHATE
17.3.2 NATURAL BONE GRAFT
17.3.2.1. XENOGRAFT
17.3.2.1.1. BOVINE-DERIVED
17.3.2.1.2. PORCINE-DERIVED
17.3.2.1.3. CHITOSAN DERIVED
17.3.2.1.4. OTHERS
17.3.2.2. ALLOGRAFT
17.3.2.2.1. FRESH OR FRESH-FROZEN BONE
17.3.2.2.1.1 BY TYPE
17.3.2.2.1.1.1. DEMINERALISED BONE ALLOGRAFT
17.3.2.2.1.1.2. OCELLULAR BONE ALLOGRAFTS
17.3.2.2.1.1.3. MACHINED ALLOGRAFTS
17.3.2.2.1.2 BY USABLITY
17.3.2.2.1.2.1. BIO-REABSORBABLE
17.3.2.2.1.2.2. NON-BIO-REABSORBABLE
17.3.2.2.2. FREEZE DRIED BONE ALLOGRAFT (FDBA)
17.3.2.2.2.1 BY TYPE
17.3.2.2.2.1.1. DEMINERALISED BONE ALLOGRAFT
17.3.2.2.2.1.2. OCELLULAR BONE ALLOGRAFTS
17.3.2.2.2.1.3. MACHINED ALLOGRAFTS
17.3.2.2.2.2 BY USABILITY
17.3.2.2.2.2.1. BIO-REABSORBABLE
17.3.2.2.2.2.2. NON-BIO-REABSORBABLE
17.3.2.3. AUTOGRAFTS
17.3.2.3.1. MANDIBULAR SYMPHYSIS
17.3.2.3.2. MANDIBULAR RAMUS
17.3.2.3.3. EXTERNAL OBLIQUE RIDGE
17.3.2.3.4. ILIAC CREST
17.3.2.3.5. PROXIMAL ULNA
17.3.2.3.6. DISTAL RADIUS
17.3.2.4. PHYTOGENIC MATERIALS
17.3.2.4.1. GUSUIBU
17.3.2.4.2. CORAL-BASED BONE SUBSTITUTES
17.3.2.4.3. MARINE ALGAE
17.3.3 LIVE OSTEOGENIC CELLS BONE SUBSTITUTES
17.3.3.1. BIOSEED-ORAL BONE
17.3.3.2. OSTEOTRANSPLANT DENT
17.3.4 GROWTH FACTORS
17.3.4.1. TRANSFORMING GROWTH FACTOR-BETA (TGF-BETA)
17.3.4.2. PLATELET-DERIVED GROWTH FACTOR (PDGF)
17.3.4.3. FIBROBLAST GROWTH FACTORS (FGF)
17.3.4.4. BONE MORPHOGENEIC PROTEIN (BMP)
17.3.4.5. OTHERS
18 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY APPLICATION
18.1 OVERVIEW
18.2 RIDGE AUGMENTATION
18.2.1 BY TYPE
18.2.1.1. DENTAL MEMBRANE
18.2.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.2.2 BY SOURCE
18.2.2.1. NATURAL
18.2.2.2. SYNTHETIC
18.3 SOCKET PRESERVATION
18.3.1 BY TYPE
18.3.1.1. DENTAL MEMBRANE
18.3.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.3.2 BY SOURCE
18.3.2.1. NATURAL
18.3.2.2. SYNTHETIC
18.4 PERIODONTAL DEFECT REGENERATION
18.4.1 BY TYPE
18.4.1.1. DENTAL MEMBRANE
18.4.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.4.2 BY SOURCE
18.4.2.1. NATURAL
18.4.2.2. SYNTHETIC
18.5 IMPLANT BONE REGENERATION
18.5.1 BY TYPE
18.5.1.1. DENTAL MEMBRANE
18.5.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.5.2 BY SOURCE
18.5.2.1. NATURAL
18.5.2.2. SYNTHETIC
18.6 SINUS LIFT
18.6.1 BY TYPE
18.6.1.1. DENTAL MEMBRANE
18.6.1.2. DENTAL BONE GRAFT SUBSTITUTE
18.6.2 BY SOURCE
18.6.2.1. NATURAL
18.6.2.2. SYNTHETIC
18.7 OTHERS
19 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY SOURCE
19.1 OVERVIEW
19.2 NATURAL
19.3 SYNTHETIC
20 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY USABILITY
20.1 OVERVIEW
20.2 RESORBABLE
20.3 NON-RESORBABLE
21 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY MECHANISM
21.1 OVERVIEW
21.2 OSTEOCONDUCTION
21.3 OSTEOINDUCTION
21.4 OSTEOPROMOTION
21.5 OSTEOGENESIS
22 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY AGE
22.1 OVERVIEW
22.2 PEDIATRICS
22.3 ADULT
22.4 GERIATRIC
23 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY POPULATION TYPE
23.1 OVERVIEW
23.2 MALE
23.3 FEMALE
24 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY END USER
24.1 OVERVIEW
24.2 HOSPITALS
24.3 DENTAL CLINICS
24.4 DENTAL LABORATORIES
24.5 AMBULATORY SURGICAL CENTERS
24.6 TRAUMA CENTER
24.7 RESEARCH AND DENTAL LABORATORIES
24.8 OTHERS
25 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY DISTRIBUTION CHANNEL
25.1 OVERVIEW
25.2 DIRECT TENDER
25.3 RETAIL SALES
25.3.1 HOSPITAL PHARMACY
25.3.2 RETAIL PHARMACY
25.3.3 ONLINE PHARMACY
25.4 OTHERS
26 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, SWOT AND DBMR ANALYSIS
27 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, COMPANY LANDSCAPE
27.1 COMPANY SHARE ANALYSIS: EUROPE
27.2 MERGERS & ACQUISITIONS
27.3 NEW PRODUCT DEVELOPMENT & APPROVALS
27.4 EXPANSIONS
27.5 REGULATORY CHANGES
27.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
28 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, BY REGION
EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
OVERVIEW (ALL SEGMENTATION PROVIDED ABOVE IS REPRESNTED IN THIS CHAPTER BY COUNTRY)
28.1 EUROPE
28.1.1 GERMANY
28.1.2 U.K.
28.1.3 ITALY
28.1.4 FRANCE
28.1.5 SPAIN
28.1.6 SWITZERLAND
28.1.7 NETHERLANDS
28.1.8 BELGIUM
28.1.9 RUSSIA
28.1.10 TURKEY
28.1.11 DENMARK
28.1.12 NORWAY
28.1.13 POLAND
28.1.14 SWEDEN
28.1.15 GREECE
28.1.16 REST OF EUROPE
28.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
29 EUROPE DENTAL MEMBRANE AND BONE GRAFT SUBSTITUTE MARKET, COMPANY PROFILE
29.1 ZIMMER BIOMET
29.1.1 COMPANY OVERVIEW
29.1.2 REVENUE ANALYSIS
29.1.3 GEOGRAPHIC PRESENCE
29.1.4 PRODUCT PORTFOLIO
29.1.5 RECENT DEVELOPMENTS
29.2 BIOVENTUS
29.2.1 COMPANY OVERVIEW
29.2.2 REVENUE ANALYSIS
29.2.3 GEOGRAPHIC PRESENCE
29.2.4 PRODUCT PORTFOLIO
29.2.5 RECENT DEVELOPMENTS
29.3 BIOHORIZONS ( HENRY SCHEIN, INC.)
29.3.1 COMPANY OVERVIEW
29.3.2 REVENUE ANALYSIS
29.3.3 GEOGRAPHIC PRESENCE
29.3.4 PRODUCT PORTFOLIO
29.3.5 RECENT DEVELOPMENTS
29.4 DENTSPLY SIRONA
29.4.1 COMPANY OVERVIEW
29.4.2 REVENUE ANALYSIS
29.4.3 GEOGRAPHIC PRESENCE
29.4.4 PRODUCT PORTFOLIO
29.4.5 RECENT DEVELOPMENTS
29.5 ZIMVIE INC.
29.5.1 COMPANY OVERVIEW
29.5.2 REVENUE ANALYSIS
29.5.3 GEOGRAPHIC PRESENCE
29.5.4 PRODUCT PORTFOLIO
29.5.5 RECENT DEVELOPMENTS
29.6 OSTEOGENICS BIOMEDICAL
29.6.1 COMPANY OVERVIEW
29.6.2 REVENUE ANALYSIS
29.6.3 GEOGRAPHIC PRESENCE
29.6.4 PRODUCT PORTFOLIO
29.6.5 RECENT DEVELOPMENTS
29.7 GEISTLICH PHARMA AG
29.7.1 COMPANY OVERVIEW
29.7.2 REVENUE ANALYSIS
29.7.3 GEOGRAPHIC PRESENCE
29.7.4 PRODUCT PORTFOLIO
29.7.5 RECENT DEVELOPMENTS
29.8 MEDTRONIC
29.8.1 COMPANY OVERVIEW
29.8.2 REVENUE ANALYSIS
29.8.3 GEOGRAPHIC PRESENCE
29.8.4 PRODUCT PORTFOLIO
29.8.5 RECENT DEVELOPMENTS
29.9 INSTITUT STRAUMANN AG
29.9.1 COMPANY OVERVIEW
29.9.2 REVENUE ANALYSIS
29.9.3 GEOGRAPHIC PRESENCE
29.9.4 PRODUCT PORTFOLIO
29.9.5 RECENT DEVELOPMENTS
29.1 BOTISS BIOMATERIALS GMBH
29.10.1 COMPANY OVERVIEW
29.10.2 REVENUE ANALYSIS
29.10.3 GEOGRAPHIC PRESENCE
29.10.4 PRODUCT PORTFOLIO
29.10.5 RECENT DEVELOPMENTS
29.11 DENTIUMUSA
29.11.1 COMPANY OVERVIEW
29.11.2 REVENUE ANALYSIS
29.11.3 GEOGRAPHIC PRESENCE
29.11.4 PRODUCT PORTFOLIO
29.11.5 RECENT DEVELOPMENTS
29.12 NOVABONE PRODUCTS, LLC. A HALMA COMPANY
29.12.1 COMPANY OVERVIEW
29.12.2 REVENUE ANALYSIS
29.12.3 GEOGRAPHIC PRESENCE
29.12.4 PRODUCT PORTFOLIO
29.12.5 RECENT DEVELOPMENTS
29.13 CGBIO
29.13.1 COMPANY OVERVIEW
29.13.2 REVENUE ANALYSIS
29.13.3 GEOGRAPHIC PRESENCE
29.13.4 PRODUCT PORTFOLIO
29.13.5 RECENT DEVELOPMENTS
29.14 REGENITY
29.14.1 COMPANY OVERVIEW
29.14.2 REVENUE ANALYSIS
29.14.3 GEOGRAPHIC PRESENCE
29.14.4 PRODUCT PORTFOLIO
29.14.5 RECENT DEVELOPMENTS
29.15 NEOSS
29.15.1 COMPANY OVERVIEW
29.15.2 REVENUE ANALYSIS
29.15.3 GEOGRAPHIC PRESENCE
29.15.4 PRODUCT PORTFOLIO
29.15.5 RECENT DEVELOPMENTS
29.16 BIOMATLANTE
29.16.1 COMPANY OVERVIEW
29.16.2 REVENUE ANALYSIS
29.16.3 GEOGRAPHIC PRESENCE
29.16.4 PRODUCT PORTFOLIO
29.16.5 RECENT DEVELOPMENTS
29.17 GRAFTYS
29.17.1 COMPANY OVERVIEW
29.17.2 REVENUE ANALYSIS
29.17.3 GEOGRAPHIC PRESENCE
29.17.4 PRODUCT PORTFOLIO
29.17.5 RECENT DEVELOPMENTS
29.18 DEPUY SYNTHES (JOHNSON & JOHNSON)
29.18.1 COMPANY OVERVIEW
29.18.2 REVENUE ANALYSIS
29.18.3 GEOGRAPHIC PRESENCE
29.18.4 PRODUCT PORTFOLIO
29.18.5 RECENT DEVELOPMENTS
29.19 BONESUPPORT AB
29.19.1 COMPANY OVERVIEW
29.19.2 REVENUE ANALYSIS
29.19.3 GEOGRAPHIC PRESENCE
29.19.4 PRODUCT PORTFOLIO
29.19.5 RECENT DEVELOPMENTS
29.2 WRIGHT MEDICAL GROUP N.V.
29.20.1 COMPANY OVERVIEW
29.20.2 REVENUE ANALYSIS
29.20.3 GEOGRAPHIC PRESENCE
29.20.4 PRODUCT PORTFOLIO
29.20.5 RECENT DEVELOPMENTS
29.21 CURASAN AG
29.21.1 COMPANY OVERVIEW
29.21.2 REVENUE ANALYSIS
29.21.3 GEOGRAPHIC PRESENCE
29.21.4 PRODUCT PORTFOLIO
29.21.5 RECENT DEVELOPMENTS
30 RELATED REPORTS
31 CONCLUSION
32 QUESTIONNAIRE
33 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.













