Europe Heparin Market
Market Size in USD million
CAGR :
% 
 USD
3,472.05 million
USD
6,586.83 million
2022
2030
USD
3,472.05 million
USD
6,586.83 million
2022
2030
| 2023 –2030 | |
| USD 3,472.05 million | |
| USD 6,586.83 million | |
|
|
|
Europe Heparin Market Analysis and Size
The rise in venous thromboembolism and cardiovascular diseases is the major factor influencing the market growth rate. Furthermore, surge in the number of applications of heparin among various surgeries including orthopaedic and heart surgeries, growing government support for the improvement of healthcare infrastructure and increase in the demand of surgeries due to the rising geriatric population are the factors that will expand the Europe heparin market.
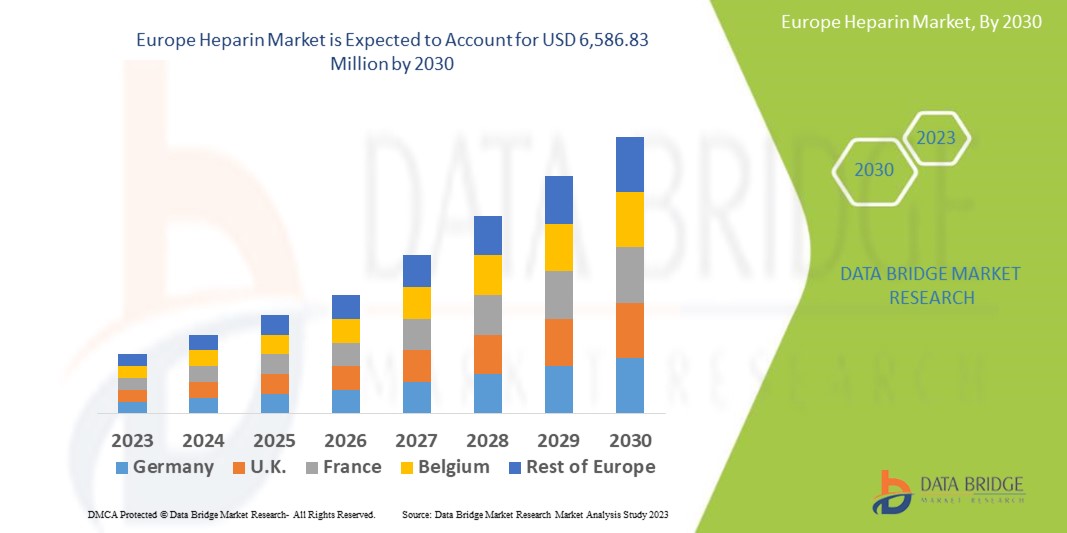
Data Bridge Market Research analyses that the Europe heparin market which was USD 3,472.05 million in 2022, is expected to reach USD 6,586.83 million by 2030, and is expected to undergo a CAGR of 5.83% during the forecast period 2023-2030. The “Unfractionated Heparin” segment dominates the product type segment of the Europe heparin market owing rising prevalence of cardiovascular diseases which drive the demand for heparin. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Heparin Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Unfractionated Heparin, Low Molecular Weight Heparin (LMWH), Ultra-Low Molecular Weight Heparin (ULMWH)), Mode of Administration (Oral, Parenteral), Source (Bovine, Porcine), Ingredients (Sodium, Calcium, Others), Availability (Raw, Processed), Treatment (Deep Vein Thrombosis, Pulmonary Embolism, Arterial Thromboembolism, Others), Application (Pre-Surgical Procedures, Post-Surgical Procedures, Kidney Dialysis, Diagnostic Tests, Others), Therapeutics (Cardiovascular, Respiratory, Oncology, Nephrology, CNS, Others), Strength (10 Unit, 100 Unit, 1000 Unit, 5000 Unit, 10000 Unit, 25000 Unit, Others), Type (Generics, Brands), Container (Bottles, Bags, Vials, Others), Packaging (Glass, Plastic), End User (Hospitals, Clinics, Homecare, Ambulatory Surgical Centres, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy and Drug Store, Online Pharmacy, Others) |
|
Countries Covered |
Germany, U.K., France, Italy, Spain, Russia, Poland, Switzerland, Netherlands, Hungary, Austria, Norway, Ireland, Turkey, Lithuania, Rest of Europe |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (United States), Teva Pharmaceutical Industries Ltd. (Israel), Sanofi (France), Pfizer Inc. (United States), Aspen Holdings (South Africa), Changzhaou Qianhong Bio-pharma Co., Ltd. (China), Eisai Co., Ltd. (Japan), Fresenius Kabi AG (Germany), Hebei Changshan Biochemical Pharmaceutical Co. Ltd. (China), Hikma Pharmaceuticals PLC (Jordan), LEO Pharma A/S (Denmark), Novartis AG (Switzerland), SARIA A/S GmbH & Co. KG (Germany), OPOCRIN S.P.A. (Italy), Bristol-Myers Squibb Company (United States), GlaxoSmithKline plc. (United Kingdom),Dr. Reddy’s Laboratories Ltd. (India), B. Braun Melsungen AG (Germany), Baxter (United States) |
|
Market Opportunities |
|
Market Definition
Heparin is a drug that prevents and treats blood clots. It's a drug that's used to treat blood coagulation abnormalities. It's also used to prevent blood from clotting after surgery, during blood transfusions, dialysis, and during taking blood samples. By releasing anti-clotting protein, it aids in the smooth flow of blood. It's referred to as an anticoagulant. It is all treated with the disseminated intravascular coagulation, deep vein thrombosis, pulmonary embolism, and arterial thromboembolism. It does not eliminate blood clots; instead, it prevents them from growing larger and creating more catastrophic complications. Heparin is a substance that comes from the mucosal tissues of killed livestock animals.
Europe Heparin Market Dynamics
Drivers
- Rising Prevalence of Cardiovascular Diseases
The increasing incidence of cardiovascular diseases, such as deep vein thrombosis (DVT), pulmonary embolism, and coronary artery diseases, is a significant driver for the heparin market. Heparin is widely used for anticoagulation therapy in these conditions, driving demand.
- Growing Surgical Procedures
Surgical procedures, including open-heart surgeries, kidney dialysis, and organ transplants, often require anticoagulation therapy to prevent blood clots. Heparin's effectiveness in reducing clotting risk during surgeries fuels its demand.
- Advancements in Heparin Production
The development of low molecular weight heparin (LMWH) with improved safety and efficacy profiles has expanded heparin usage. LMWH is preferred in many clinical scenarios and is a driving factor in the market.
Opportunities
- Increasing Research and Development
Ongoing research into heparin derivatives and alternatives, as well as biosynthetic heparin production, presents opportunities for innovation in the market. These advancements may lead to safer and more effective anticoagulant therapies
- Growing Awareness of Thrombosis
Raising awareness about the risks of thrombosis and the benefits of anticoagulation therapy can drive market growth. Public health campaigns and educational initiatives can contribute to increased heparin use.
Restraints/ Challenges
- Regulatory Challenges
Stringent regulatory requirements for heparin production, especially in terms of quality and safety, can pose challenges for manufacturers. Compliance with these regulations is essential but can be costly and time-consuming.
- Competition from Novel Anticoagulants
Novel oral anticoagulants (NOACs), such as dabigatran and rivaroxaban, offer alternatives to traditional heparin therapy. These drugs are easier to administer and have a lower risk of HIT. Market competition from NOACs presents a challenge for heparin products.
This Europe heparin market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Europe heparin market Contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In May 2023, according to European Bioinformatics Institute Fibroblast growth factors are heparin-binding proteins, and interactions with cell surface-bound heparan sulphate proteoglycans have been found to be critical for FGF signaling
Europe Heparin Market Scope
Europe heparin market is segmented on the basis of product type, mode of administration, source, ingredients, availability, treatment, application, therapeutics, strength, type, container, packaging, end user and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries, and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
Product Type
- Unfractionated Heparin
- Low Molecular Weight Heparin (LMWH)
- Ultra-Low Molecular Weight Heparin (ULMWH)
Mode of Administration
- Oral
- Parenteral
Source
- Bovine
- Porcine
Ingredients
- Sodium
- Calcium
- Others
Availability
- Raw
- Processed
Treatment
- Deep Vein Thrombosis
- Pulmonary Embolism
- Arterial Thromboembolism
- Others
Application
- Pre-Surgical Procedures
- Post-Surgical Procedures
- Kidney Dialysis
- Diagnostic Tests
- Others
Therapeutics
- Cardiovascular
- Respiratory
- Oncology
- Nephrology
- CNS
- Others
Strength
- 10 unit
- 100 unit
- 1000 unit
- 5000 unit
- 10000 unit
- 25000 unit
- Others
Type
- Generics
- Brands
Container
- Bottles
- Bags
- Vials
- Others
Packaging
- Glass
- Plastic
End User
- Hospital
- Clinics
- Homecare
- Ambulatory Surgical Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Drug Store
- Online Pharmacy
- Others
Europe Heparin Market Regional Analysis/Insights
Europe heparin market is analyzed and market size information is provided by the country, product type, mode of administration, source, ingredients, availability, treatment, application, therapeutics, strength, type, container, packaging, end user and distribution channel as referenced above.
The countries covered in this Europe heparin market report are Germany, U.K., France, Italy, Spain, Russia, Poland, Switzerland, Netherlands, Hungary, Austria, Norway, Ireland, Turkey, Lithuania, and Rest of Europe.
Germany dominates the Europe heparin market due to the presence of major key players, and developing healthcare infrastructure in this region. France and U.K. are expected to grow during the forecast period of 2023-2030 due to the increasing research and development activities, and growing prevalence of chronic disorders in this region.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Heparin Market Share Analysis
Europe heparin market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, company strengths and weaknesses, product launch, clinical trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies’ focus related to Europe heparin market research.
Some of the major players operating in the Europe heparin market are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (United States)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Pfizer Inc. (United States)
- Aspen Holdings (South Africa)
- Changzhaou Qianhong Bio-pharma Co., Ltd. (China)
- Eisai Co., Ltd. (Japan)
- Fresenius Kabi AG (Germany)
- Hebei Changshan Biochemical Pharmaceutical Co. Ltd. (China)
- Hikma Pharmaceuticals PLC (Jordan)
- LEO Pharma A/S (Denmark)
- Novartis AG (Switzerland)
- SARIA A/S GmbH & Co. KG (Germany)
- OPOCRIN S.P.A. (Italy)
- Bristol-Myers Squibb Company (United States)
- GlaxoSmithKline plc. (United Kingdom)
- Dr. Reddy’s Laboratories Ltd. (India)
- B. Braun Melsungen AG (Germany)
- Baxter (United States)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.