Europe Infection Control Market
Market Size in USD Million
CAGR :
% 
 USD
8,934.67 Million
USD
26,580.03 Million
2022
2030
USD
8,934.67 Million
USD
26,580.03 Million
2022
2030
| 2023 –2030 | |
| USD 8,934.67 Million | |
| USD 26,580.03 Million | |
|
|
|
|
Europe Infection Control Market Analysis and Size
According to the data provided by the WHO, Acute Respiratory Diseases (ARDs) are one of the main reasons for the high mortality rates experienced. Every year, ARDs are responsible for over 4.0 million deaths. The data given above demonstrate the critical necessity for strict prevention and control. The majority of all hospital-acquired infections are caused by illnesses such as pneumonia and infections of the bloodstream, urinary system, surgical site, and MRSA. The infections may be transmitted directly or indirectly from one person to another through contaminated or unsterilized surgical or medical equipment used to treat a patient or from a contaminated environment in a healthcare facility.
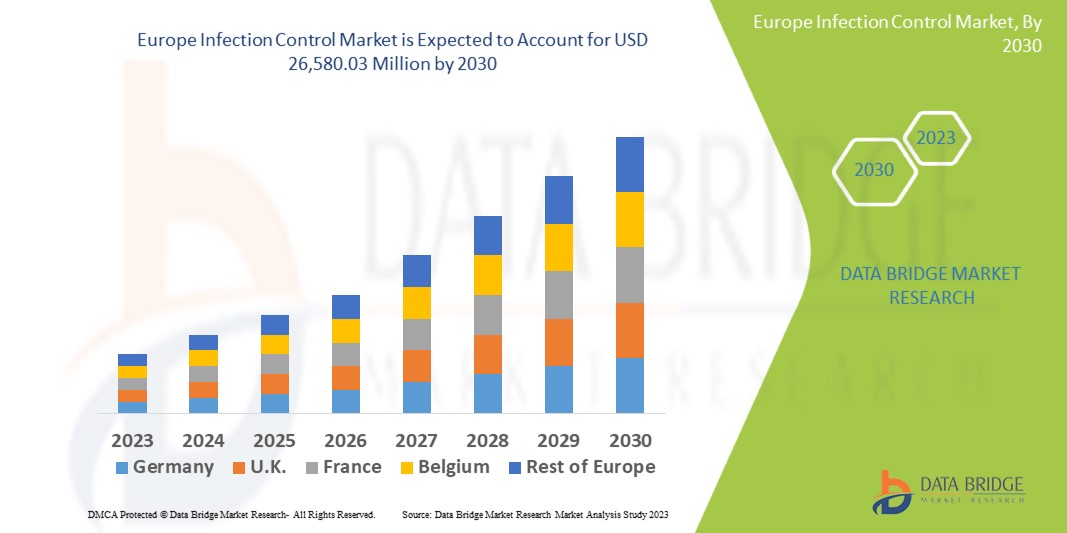
Data Bridge Market Research analyses that the infection control market which is USD 8,934.67 million in 2022, is expected to reach USD 26,580.03 million by 2030, at a CAGR of 14.60% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Infection Control Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Sterilization, Cleaning and Disinfection, Personal Protective Barriers, Endoscope Reprocessing, Anti-Microbial Surfaces, Other Infection Control), Application (Surgical Instruments, Endoscopes, Ultrasound Probes, Others), Distribution Channel (Direct Tender, Retail Sales, Third Party Distributors), End User (Hospitals, Clinics, Medical Device Companies, Pharmaceutical and Biotechnology Companies, Laboratories, Others) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
STERIS plc (U.S.), Sotera Health Company (U.S.), Getinge AB (Sweden), Advanced Sterilization Products (ASP) (U.S.), Ecolab Inc. (U.S.), 3M (U.S.), MATACHANA GROUP (Spain), MMM Group (Germany), Belimed AG (Switzerland), Reckitt Benckiser (U.K.), Metrex Research LLC (U.K.), Miele Group (Germany), Pal International (U.K.), MELAG Medizintechnik GmbH & Co. KG (Germany), Contec, Inc. (U.S.), MEDALKAN (Greece), Systec GmbH (Germany), C.B.M. S.r.l. Medical Equipment (Italy), Continental Equipment Company (U.S.), and BGS Beta-Gamma-Service GmbH & Co. KG (Germany) |
|
Market Opportunities |
|
Market Definition
The process of infection control is used in the healthcare sector to stop or prevent the spread of infections. The activity of infection control involves getting rid of bacteria from various surfaces and things. This procedure makes it possible for people to avoid disease transmission and pollution. A scientific strategy called infection control is intended to stop the harm infections can do to patients and healthcare professionals. In all healthcare settings, infection control is necessary to stop the spread of infectious diseases. It can be described as a set of generally accepted suggestions used to decrease the danger of infectious agents being transmitted by environmental or bodily fluids.
Europe Infection Control Market Dynamics
Drivers
- Rising government organizations
In the forecast period, the market is expected to develop as a result of the rising involvement of governmental organizations in the publication of recommendations to spread knowledge about practical preventative strategies. For instance, the World Health Organization (WHO) has issued guidelines in healthcare for the prevention and control of pandemic and epidemic-prone acute respiratory diseases. The guidelines cover everything from standard precautions such as hand hygiene and the use of personal protective equipment to disinfection and sterilization procedures.
- Increase in hospitalization
According to a WHO guidelines on HAI prevention, the increase in hospitalization duration with surgical infections was found to be approximately eight days. These extended stays are expected to contribute significantly to the overall costs incurred during the hospitalization, raising the clinical urgency for implementing infection prevention measures. It is assumed that a prolonged hospital stay is also not cost effective for hospitals and healthcare payers due to the excessive use of resources to treat the acquired infection. These additional costs are primarily caused by increased drug usage, additional diagnostic studies, and laboratory equipment, resulting in a resource allocation imbalance.
Opportunities
- Increasing use of e-beam sterilization
Beta radiation, often known as electron-beam irradiation, is frequently used to sterilize medical equipment and pharmaceutical packaging. It works by continuously passing electrons through the items being sterilized. The benefit of this treatment is that it offers quick-turn terminal sterilization using straightforward, hygienic on/off technology. The product can be distributed right away because no residuals or radioactive or carcinogenic gas sources are required. The market for infection control is anticipated to have considerable expansion during the forecast period as a result of the expanding applications of e-beam sterilization and its adoption among various end-user sectors.
Restraints/Challenges
- Concerns about the safety of the processed instruments
Concerns about the safety of the processed instruments used in surgeries are extremely dangerous for patients as well as healthcare workers or professionals, resulting in the spread of infection and acting as a restraint factor hampered the growth of the infection control market.
This infection control market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the infection control market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2020, Getinge AB launched superfast indicators to further ensure patient safety. The new Superfast 20 indicators are ISO 11138 compliant and compatible with most steam and hydrogen peroxide process sterilizers. This product launch will assist the company in increasing its market share in the global patient safety market.
- In 2019, Cardinal Health announced the acquisition of mscripts, a company that provides patient adherence and engagement solutions through an innovative, user-friendly mobile and web-based health management platform. This will assist the company in promoting patient adherence to prescriptions and improving overall health outcomes.
Europe Infection Control Market Scope
The infection control market is segmented on the basis of product, application, distribution channel and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Sterilization
- Cleaning and Disinfection
- Personal Protective Barriers
- Endoscope Reprocessing
- Anti-Microbial Surfaces
- Other Infection Control
Application
- Surgical Instruments
- Endoscopes
- Ultrasound Probes
- Others
Distribution Channel
- Direct Tender
- Retail Sales
- Third Party Distributors
End User
- Hospitals
- Clinics
- Medical Device Companies
- Pharmaceutical and Biotechnology Companies
- Laboratories
- Others
Infection Control Market Regional Analysis/Insights
The infection control market is analyzed and market size insights and trends are provided by country, product, application, distribution channel and end user as referenced above.
The countries covered in the infection control market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
France is expected to grow with the most promising growth rate in the forecast period of 2023 to 2030 due to rising healthcare spending and an increase in infectious disease prevalence in the area.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The infection control market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for infection control market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the infection control market. The data is available for historic period 2011-2021.
Competitive Landscape and Infection Control Market Share Analysis
The infection control market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to infection control market.
Some of the major players operating in the infection control market are:
- STERIS plc (U.S.)
- Sotera Health Company (U.S.)
- Getinge AB (Sweden)
- Advanced Sterilization Products (ASP) (U.S.)
- Ecolab Inc. (U.S.)
- 3M (U.S.)
- MATACHANA GROUP (Spain)
- MMM Group (Germany)
- Belimed AG (Switzerland)
- Reckitt Benckiser (U.K.)
- Metrex Research LLC (U.K.)
- Miele Group (Germany)
- Pal International (U.K.)
- MELAG Medizintechnik GmbH & Co. KG (Germany)
- Contec, Inc. (U.S.)
- MEDALKAN (Greece)
- Systec GmbH (Germany)
- C.B.M. S.r.l. Medical Equipment (Italy)
- Continental Equipment Company (U.S.)
- BGS Beta-Gamma-Service GmbH & Co. KG (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INFECTION CONTROL MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE INFECTION CONTROL MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE INFECTION CONTROL MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 EUROPE INFECTION CONTROL MARKET, BY PRODUCT AND SERVICE
16.1 OVERVIEW
16.2 PRODUCTS
16.2.1 STERILIZATION PRODUCTS
16.2.1.1. STERILIZATION EQUIPMENT
16.2.1.1.1. HEAT STERILIZATION EQUIPMENT
16.2.1.1.1.1 MOIST HEAT STERILIZERS
16.2.1.1.1.1.1. MARKET SHARE (USD)
16.2.1.1.1.1.2. MARKET VOLUME (UNIT)
16.2.1.1.1.1.3. AVERAGE SELLING PRICE (USD)
16.2.1.1.1.2 DRY HEAT STERILIZERS
16.2.1.1.2. LOW TEMPERATURE STERILIZATION
16.2.1.1.2.1 HYDROGEN PEROXIDE STERILIZATION
16.2.1.1.2.2 ETHYLENE OXIDE STERILIZATION (ETO)
16.2.1.1.2.3 FORMALDEHYDE STERILIZATION
16.2.1.1.2.4 OTHER LOW-TEMPERATURE STERILIZATION
16.2.1.1.3. RADIATION STERILIZATION
16.2.1.1.4. FILTRATION STERILIZATION
16.2.1.1.5. OTHER EQUIPMENT
16.2.1.2. CONSUMABLES AND ACCESSORIES
16.2.1.2.1. STERILIZATION INDICATORS
16.2.1.2.2. CHEMICAL INDICATORS
16.2.1.2.3. BIOLOGICAL INDICATORS
16.2.1.2.4. PACKAGING ACCESSORIES
16.2.1.2.5. OTHERS
16.2.2 CLEANING AND DISINFECTION PRODUCTS
16.2.2.1. DISINFECTANTS
16.2.2.1.1. BY PRODUCT TYPE
16.2.2.1.1.1 HAND DISINFECTANTS
16.2.2.1.1.1.1. BY TYPE
A. ALCOHOL BASED
I. ETHANOL (ETHYL ALCOHOL)
II. ISOPROPYL ALCOHOL (ISOPROPANOL OR 2-PROPANOL)
III. OTHERS
B. ALCOHOL FREE
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. HYDROXYACETOPHENONE
IV. IODINE
V. TRICLOSAN
VI. OTHERS
16.2.2.1.1.1.2. BY DOSAGE
A. FOAM
B. GEL
C. LIQUID
D. WIPES
E. OTHERS
16.2.2.1.1.1.3. BY EPA CLASSIFICATION
A. LOW-LEVEL DISINFECTANTS
I. QUATERNARY AMMONIUM COMPOUNDS
II. IODOPHORS
III. PHENOLICS
B. INTERMEDIATE-LEVEL DISINFECTANTS
I. PHENOLS
II. CHLORINE COMPOUNDS
III. OTHERS
C. HIGH-LEVEL DISINFECTANTS
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. SUPEROXIDIZED WATER
IV. OTHERS
16.2.2.1.1.2 SURFACE DISINFECTANTS
16.2.2.1.1.2.1. ALCOHOL BASED
A. ETHANOL (ETHYL ALCOHOL)
B. ISOPROPYL ALCOHOL (ISOPROPANOL OR 2-PROPANOL)
16.2.2.1.1.2.2. ALCOHOL FREE
A. CHLORHEXIDINE
B. CHLOROXYLENOL
C. HYDROXYACETOPHENONE
D. IODINE
E. TRICLOSAN
F. OTHERS
16.2.2.1.1.2.3. BY DOSAGE
A. FOAM
B. GEL
C. LIQUID
D. WIPES
E. OTHERS
16.2.2.1.1.2.4. BY EPA CLASSIFICATION
A. LOW-LEVEL DISINFECTANTS
I. QUATERNARY AMMONIUM COMPOUNDS
II. IODOPHORS
III. PHENOLICS
B. INTERMEDIATE-LEVEL DISINFECTANTS
I. PHENOLS
II. CHLORINE COMPOUNDS
III. OTHERS
C. HIGH-LEVEL DISINFECTANTS
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. SUPEROXIDIZED WATER
IV. OTHERS
16.2.2.1.1.3 SKIN DISINFECTANTS
16.2.2.1.1.3.1. ALCOHOL BASED
16.2.2.1.1.3.2. ETHANOL (ETHYL ALCOHOL)
16.2.2.1.1.3.3. ISOPROPYL ALCOHOL (ISOPROPANOL OR 2-PROPANOL)
A. ALCOHOL FREE
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. HYDROXYACETOPHENONE
IV. IODINE
V. TRICLOSAN
VI. OTHERS
16.2.2.1.1.3.4. BY DOSAGE
A. FOAM
B. GEL
C. LIQUID
D. WIPES
E. OTHERS
16.2.2.1.1.3.5. BY EPA CLASSIFICATION
A. LOW-LEVEL DISINFECTANTS
I. QUATERNARY AMMONIUM COMPOUNDS
II. IODOPHORS
III. PHENOLICS
B. INTERMEDIATE-LEVEL DISINFECTANTS
I. PHENOLS
II. CHLORINE COMPOUNDS
III. OTHERS
C. HIGH-LEVEL DISINFECTANTS
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. SUPEROXIDIZED WATER
IV. OTHERS
16.2.2.1.1.4 INSTRUMENT DISINFECTANTS
A. ALCOHOL BASED
I. ETHANOL (ETHYL ALCOHOL)
II. ISOPROPYL ALCOHOL (ISOPROPANOL OR 2-PROPANOL)
B. ALCOHOL FREE
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. HYDROXYACETOPHENONE
IV. IODINE
V. TRICLOSAN
VI. OTHERS
16.2.2.1.1.4.2. BY DOSAGE
A. FOAM
B. GEL
C. LIQUID
D. WIPES
E. OTHERS
16.2.2.1.1.4.3. BY EPA CLASSIFICATION
A. LOW-LEVEL DISINFECTANTS
I. QUATERNARY AMMONIUM COMPOUNDS
II. IODOPHORS
III. PHENOLICS
B. INTERMEDIATE-LEVEL DISINFECTANTS
I. PHENOLS
II. CHLORINE COMPOUNDS
III. OTHERS
C. HIGH-LEVEL DISINFECTANTS
I. CHLORHEXIDINE
II. CHLOROXYLENOL
III. SUPEROXIDIZED WATER
IV. OTHERS
16.2.2.2. CLEANING AND DISINFECTION EQUIPMENT
16.2.2.2.1. WASHER DISINFECTORS
16.2.2.2.1.1 SINGLE-CHAMBER WASHERS
16.2.2.2.1.2 MULTI-CHAMBER WASHERS
16.2.2.2.1.3 GENERATION WASHER/DISINFECTORS
16.2.2.2.2. FLUSHER DISINFECTORS
16.2.2.2.2.1 FRONT LOADING
16.2.2.2.2.2 TOP LOADING
16.2.2.2.3. UV-RAY DISINFECTORS
16.2.2.2.3.1 BY TYPE OF RAYS
16.2.2.2.3.1.1. UV- RAY A
16.2.2.2.3.1.2. UV-RAY B
16.2.2.2.3.2 BY TYPE
16.2.2.2.3.2.1. FLOOR STANDING
16.2.2.2.3.2.2. BENCH TYPE
16.2.2.2.4. ULTRASONIC CLEANERS
16.2.2.2.4.1 BY TYPE
16.2.2.2.4.1.1. EQUIPMENTS
16.2.2.2.4.1.2. VIALS
16.2.2.2.4.1.3. BOTTLES
16.2.2.2.4.1.4. SYRINGES
16.2.2.2.4.2 BY ACCESSORIES
16.2.2.2.4.2.1. SINK
16.2.2.2.4.2.2. CLEANSER
16.2.2.2.5. OTHER CLEANING AND DISINFECTION EQUIPMENT
16.2.2.3. LUBRICANTS AND CLEANING SOLUTIONS
16.2.2.3.1. HYPOCHLORITE
16.2.2.3.2. ALCOHOLS
16.2.2.3.3. CHLORINE DIOXIDE
16.2.2.3.4. HYDROGEN PEROXIDE & PERACETIC ACID
16.2.2.3.5. IODOPHOR DISINFECTANT
16.2.2.3.6. QUATERNARY AMMONIUM COMPOUNDS
16.2.2.4. DISINFECTION AND CLEANING ACCESSORIES
16.2.2.4.1. CLEANING TROLLEYS
16.2.2.4.2. CLEANING STATIONS
16.2.3 PERSONAL PROTECTIVE BARRIERS
16.2.3.1. MEDICAL NONWOVENS
16.2.3.1.1. SURGICAL DRAPES
16.2.3.1.1.1 GENERAL SURGERY
16.2.3.1.1.2 ORTHOPEDIC SURGERY
16.2.3.1.1.3 CARDIAC SURGERY
16.2.3.1.1.4 FOR GYNECOLOGICAL SURGERY
16.2.3.1.1.5 UROLOGIC SURGERY
16.2.3.1.1.6 ANGIOGRAPHY
16.2.3.1.1.7 DENTAL SURGERY
16.2.3.1.1.8 CRANIOTOMY
16.2.3.1.1.9 OPHTHALMIC SURGERY
16.2.3.1.1.10 ENT SURGERY
16.2.3.1.1.11 OTHERS
16.2.3.1.2. SURGICAL GOWNS
16.2.3.1.2.1 BY USE
16.2.3.1.2.1.1. HEALTHCARE STAFF
16.2.3.1.2.1.2. PATIENT / VISITOR
16.2.3.1.2.1.3. LABORATORY STAFF
16.2.3.1.2.1.4. OTHERS
16.2.3.1.2.2 BY MATERIAL
16.2.3.1.2.2.1. POLYPROPYLENE
16.2.3.1.2.2.2. POLYETHYLENE
16.2.3.1.2.2.3. POLYESTER
16.2.3.1.2.2.4. COTTON
16.2.3.1.2.2.5. VISCOSE
16.2.3.1.2.2.6. OTHERS
16.2.3.1.3. FACE MASKS
16.2.3.1.3.1 FACIAL VENTILATION MASKS
16.2.3.1.3.2 FACIAL LARYNGEAL MASKS
16.2.3.1.3.3 HALF-MASKS FACE-SHIELDS
16.2.3.1.3.4 FACIAL ANESTHESIA MASKS
16.2.3.1.3.5 FACIAL NEBULIZATION MASKS
16.2.3.1.3.6 FACIAL RESUSCITATION MASKS
16.2.3.1.3.7 OTHERS
16.2.3.2. FACE SHIELDS
16.2.3.2.1. FULL FACE
16.2.3.2.2. HALF MASK
16.2.3.3. GOGGLES
16.2.3.3.1. SAFETY GLASSES
16.2.3.3.2. VIRTUAL REALITY GOGGLES
16.2.3.3.3. FRENZEL GOGGLES
16.2.3.4. OTHERS
16.2.4 ENDOSCOPE REPROCESSING PRODUCTS
16.2.4.1. ENDOSCOPE REPROCESSING
16.2.4.1.1. ELECTRODES
16.2.4.1.2. STORAGE DEVICES
16.2.4.1.3. CONNECTORS
16.2.4.1.4. OTHERS
16.2.4.2. ENDOSCOPE REPROCESSING EQUIPMENT
16.2.4.2.1. AUTOMATED ENDOSCOPE REPROCESSORS
16.2.4.2.2. ENDOSCOPE TRACKING SYSTEMS
16.2.4.2.3. OTHER ENDOSCOPE REPROCESSING PRODUCTS
16.2.5 CLEANERS & DETERGENTS
16.2.5.1. CLEANERS
16.2.5.1.1. LIQUID SOLUTIONS
16.2.5.1.2. FOAM-BASED CLEANERS
16.2.5.1.3. GEL-BASED CLEANERS
16.2.5.2. DETERGENTS
16.2.5.2.1. ENZYMATIC DETERGENTS
16.2.5.2.2. NEUTRAL DETERGENTS
16.2.5.2.3. ACIDIC DETERGENTS
16.2.5.2.4. ALKALINE DETERGENTS
16.2.6 DISINFECTANT SPRAYS
16.2.6.1. FLAVOURED
16.2.6.2. NON-FLAVOURED
16.2.7 OTHER INFECTION CONTROL PRODUCTS
16.3 SERVICES
16.3.1 CONTRACT SERVICES
16.3.2 INHOUSE SERVICES
17 EUROPE INFECTION CONTROL MARKET, BY APPLICATION
17.1 OVERVIEW
17.2 SURGICAL INSTRUMENTS
17.3 ENDOSCOPES
17.4 ULTRASOUND PROBES
17.5 OTHERS
18 EUROPE INFECTION CONTROL MARKET, BY END USER
18.1 OVERVIEW
18.2 CLINICS
18.3 HOSPITALS
18.3.1 BY FACILITY
18.3.1.1. CSSD
18.3.1.2. PATIENT ROOM
18.3.1.3. OPERATING ROOM
18.3.1.4. OTHERS
18.3.2 BY TYPE
18.3.2.1. PUBLIC
18.3.2.2. PRIVATE
18.3.3 BY LEVEL
18.3.3.1. TIER 1
18.3.3.2. TIER 2
18.3.3.3. TIER 3
18.4 MEDICAL DEVICE COMPANIES
18.5 PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES
18.6 RESEARCH LABORA
18.7 DIAGNOSTIC LABORATORIES
18.8 OTHERS
19 EUROPE INFECTION CONTROL MARKET, BY DISTRIBUTION CHANNEL
19.1 OVERVIEW
19.2 DIRECT TENDER
19.3 RETAIL SALES
19.3.1 OFFLINE
19.3.2 ONLINE
19.4 OTHERS
20 EUROPE INFECTION CONTROL MARKET, COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: EUROPE
20.2 MERGERS & ACQUISITIONS
20.3 NEW PRODUCT DEVELOPMENT & APPROVALS
20.4 EXPANSIONS
20.5 REGULATORY CHANGES
20.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
21 EUROPE INFECTION CONTROL MARKET, BY REGION
21.1 EUROPE INFECTION CONTROL MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
21.1.1 EUROPE
21.1.1.1. GERMANY
21.1.1.2. FRANCE
21.1.1.3. U.K.
21.1.1.4. ITALY
21.1.1.5. SPAIN
21.1.1.6. RUSSIA
21.1.1.7. TURKEY
21.1.1.8. BELGIUM
21.1.1.9. NETHERLANDS
21.1.1.10. SWITZERLAND
21.1.1.11. REST OF EUROPE
21.1.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
22 EUROPE INFECTION CONTROL MARKET, SWOT & DBMR ANALYSIS
23 EUROPE INFECTION CONTROL MARKET, COMPANY PROFILE
23.1 3M
23.1.1 COMPANY OVERVIEW
23.1.2 REVENUE ANALYSIS
23.1.3 GEOGRAPHIC PRESENCE
23.1.4 PRODUCT PORTFOLIO
23.1.5 RECENT DEVELOPMENTS
23.2 ASP INTERNATIONAL GMBH
23.2.1 COMPANY OVERVIEW
23.2.2 REVENUE ANALYSIS
23.2.3 GEOGRAPHIC PRESENCE
23.2.4 PRODUCT PORTFOLIO
23.2.5 RECENT DEVELOPMENTS
23.3 SOTERA HEALTH
23.3.1 COMPANY OVERVIEW
23.3.2 REVENUE ANALYSIS
23.3.3 GEOGRAPHIC PRESENCE
23.3.4 PRODUCT PORTFOLIO
23.3.5 RECENT DEVELOPMENTS
23.4 THE CLOROX COMPANY.
23.4.1 COMPANY OVERVIEW
23.4.2 REVENUE ANALYSIS
23.4.3 GEOGRAPHIC PRESENCE
23.4.4 PRODUCT PORTFOLIO
23.4.5 RECENT DEVELOPMENTS
23.5 TERRAGENE
23.5.1 COMPANY OVERVIEW
23.5.2 REVENUE ANALYSIS
23.5.3 GEOGRAPHIC PRESENCE
23.5.4 PRODUCT PORTFOLIO
23.5.5 RECENT DEVELOPMENTS
23.6 CISA
23.6.1 COMPANY OVERVIEW
23.6.2 REVENUE ANALYSIS
23.6.3 GEOGRAPHIC PRESENCE
23.6.4 PRODUCT PORTFOLIO
23.6.5 RECENT DEVELOPMENTS
23.7 SCICAN LTD. (COLTENE)
23.7.1 COMPANY OVERVIEW
23.7.2 REVENUE ANALYSIS
23.7.3 GEOGRAPHIC PRESENCE
23.7.4 PRODUCT PORTFOLIO
23.7.5 RECENT DEVELOPMENTS
23.8 OLYMPUS CORPORATION
23.8.1 COMPANY OVERVIEW
23.8.2 REVENUE ANALYSIS
23.8.3 GEOGRAPHIC PRESENCE
23.8.4 PRODUCT PORTFOLIO
23.8.5 RECENT DEVELOPMENTS
23.9 B. BRAUN SE
23.9.1 COMPANY OVERVIEW
23.9.2 REVENUE ANALYSIS
23.9.3 GEOGRAPHIC PRESENCE
23.9.4 PRODUCT PORTFOLIO
23.9.5 RECENT DEVELOPMENTS
23.1 STRYKER
23.10.1 COMPANY OVERVIEW
23.10.2 REVENUE ANALYSIS
23.10.3 GEOGRAPHIC PRESENCE
23.10.4 PRODUCT PORTFOLIO
23.10.5 RECENT DEVELOPMENTS
23.11 CARDINAL HEALTH
23.11.1 COMPANY OVERVIEW
23.11.2 REVENUE ANALYSIS
23.11.3 GEOGRAPHIC PRESENCE
23.11.4 PRODUCT PORTFOLIO
23.11.5 RECENT DEVELOPMENTS
23.12 ZIMMER BIOMET
23.12.1 COMPANY OVERVIEW
23.12.2 REVENUE ANALYSIS
23.12.3 GEOGRAPHIC PRESENCE
23.12.4 PRODUCT PORTFOLIO
23.12.5 RECENT DEVELOPMENTS
23.13 STERIS PLC.
23.13.1 COMPANY OVERVIEW
23.13.2 REVENUE ANALYSIS
23.13.3 GEOGRAPHIC PRESENCE
23.13.4 PRODUCT PORTFOLIO
23.13.5 RECENT DEVELOPMENTS
23.14 GETINGE AB
23.14.1 COMPANY OVERVIEW
23.14.2 REVENUE ANALYSIS
23.14.3 GEOGRAPHIC PRESENCE
23.14.4 PRODUCT PORTFOLIO
23.14.5 RECENT DEVELOPMENTS
23.15 ECOLAB
23.15.1 COMPANY OVERVIEW
23.15.2 REVENUE ANALYSIS
23.15.3 GEOGRAPHIC PRESENCE
23.15.4 PRODUCT PORTFOLIO
23.15.5 RECENT DEVELOPMENTS
23.16 MATACHANA GROUP
23.16.1 COMPANY OVERVIEW
23.16.2 REVENUE ANALYSIS
23.16.3 GEOGRAPHIC PRESENCE
23.16.4 PRODUCT PORTFOLIO
23.16.5 RECENT DEVELOPMENTS
23.17 MMM GROUP
23.17.1 COMPANY OVERVIEW
23.17.2 REVENUE ANALYSIS
23.17.3 GEOGRAPHIC PRESENCE
23.17.4 PRODUCT PORTFOLIO
23.17.5 RECENT DEVELOPMENTS
23.18 BELIMED AG (METALL ZUG)
23.18.1 COMPANY OVERVIEW
23.18.2 REVENUE ANALYSIS
23.18.3 GEOGRAPHIC PRESENCE
23.18.4 PRODUCT PORTFOLIO
23.18.5 RECENT DEVELOPMENTS
23.19 METREX RESEARCH, LLC.
23.19.1 COMPANY OVERVIEW
23.19.2 REVENUE ANALYSIS
23.19.3 GEOGRAPHIC PRESENCE
23.19.4 PRODUCT PORTFOLIO
23.19.5 RECENT DEVELOPMENTS
23.2 RECKITT BENCKISER GROUP PLC
23.20.1 COMPANY OVERVIEW
23.20.2 REVENUE ANALYSIS
23.20.3 GEOGRAPHIC PRESENCE
23.20.4 PRODUCT PORTFOLIO
23.20.5 RECENT DEVELOPMENTS
23.21 STEELCO S.P.A. (MIELE)
23.21.1 COMPANY OVERVIEW
23.21.2 REVENUE ANALYSIS
23.21.3 GEOGRAPHIC PRESENCE
23.21.4 PRODUCT PORTFOLIO
23.21.5 RECENT DEVELOPMENTS
23.22 PAL INTERNATIONAL
23.22.1 COMPANY OVERVIEW
23.22.2 REVENUE ANALYSIS
23.22.3 GEOGRAPHIC PRESENCE
23.22.4 PRODUCT PORTFOLIO
23.22.5 RECENT DEVELOPMENTS
23.22.6 COMPANY OVERVIEW
23.22.7 REVENUE ANALYSIS
23.22.8 GEOGRAPHIC PRESENCE
23.22.9 PRODUCT PORTFOLIO
23.22.10 RECENT DEVELOPMENTS
23.23 MELAG MEDIZINTECHNIK GMBH & CO. KG
23.23.1 COMPANY OVERVIEW
23.23.2 REVENUE ANALYSIS
23.23.3 GEOGRAPHIC PRESENCE
23.23.4 PRODUCT PORTFOLIO
23.23.5 RECENT DEVELOPMENTS
23.24 CONTEC, INC.
23.24.1 COMPANY OVERVIEW
23.24.2 REVENUE ANALYSIS
23.24.3 GEOGRAPHIC PRESENCE
23.24.4 PRODUCT PORTFOLIO
23.24.5 RECENT DEVELOPMENTS
23.25 MEDALKAN
23.25.1 COMPANY OVERVIEW
23.25.2 REVENUE ANALYSIS
23.25.3 GEOGRAPHIC PRESENCE
23.25.4 PRODUCT PORTFOLIO
23.25.5 RECENT DEVELOPMENTS
23.26 C.B.M. S.R.L. MEDICAL EQUIPEMENT
23.26.1 COMPANY OVERVIEW
23.26.2 REVENUE ANALYSIS
23.26.3 GEOGRAPHIC PRESENCE
23.26.4 PRODUCT PORTFOLIO
23.26.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
24 RELATED REPORTS
25 CONCLUSION
26 QUESTIONNAIRE
27 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.