Europe Interventional Neurology Market
Market Size in USD Billion
CAGR :
% 
 USD
2.37 Billion
USD
3.45 Billion
2024
2032
USD
2.37 Billion
USD
3.45 Billion
2024
2032
| 2025 –2032 | |
| USD 2.37 Billion | |
| USD 3.45 Billion | |
|
|
|
|
Interventional Neurology Market Size
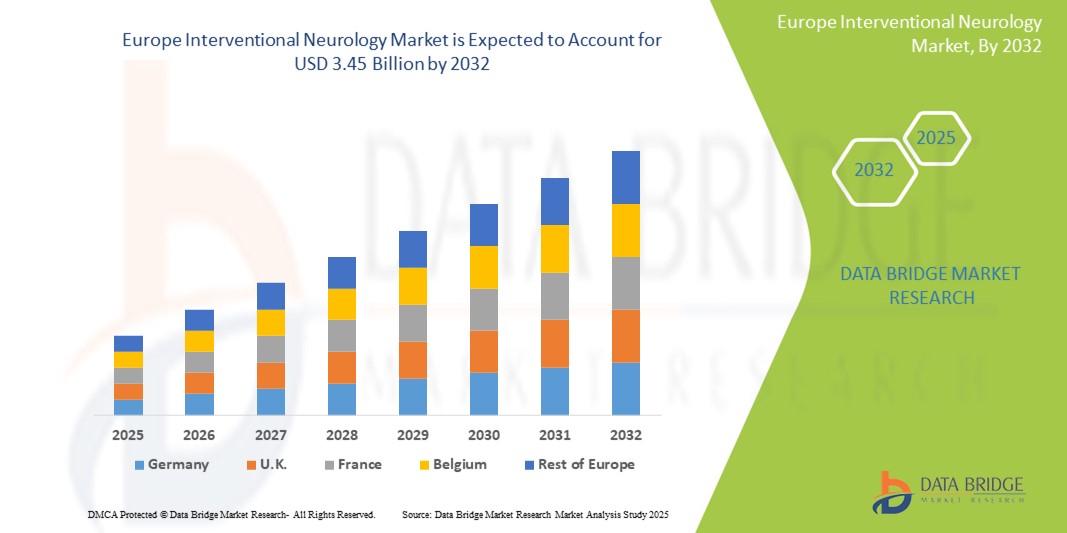
- The Europe Interventional Neurology market size was valued at USD 2.37 billion in 2024 and is expected to reach USD 3.45 billion by 2032, at a CAGR of 4.8% during the forecast period
- The Europe Interventional Neurology market encompasses a broad range of advanced imaging The Europe Interventional Neurology market encompasses a broad range of minimally invasive medical devices and procedures used to diagnose and treat disorders of the brain, spine, and peripheral nervous system. These include ischemic and hemorrhagic stroke, cerebral aneurysms, arteriovenous malformations (AVMs), and intracranial stenosis. The growing prevalence of neurovascular diseases and aging population across Europe significantly contributes to the market's expansion.
- Key interventional devices in this market include neurovascular thrombectomy devices, embolic coils, stents, flow diverters, balloon catheters, and intrasaccular devices. These tools are commonly employed in neurointerventional suites within hospitals, stroke centers, and specialized neurology clinics throughout the region. Advanced technologies like image-guided navigation, real-time 3D visualization, and robotic-assisted interventions are increasingly integrated into practice.
Interventional Neurology Market Analysis
- The Europe Interventional Neurology market is experiencing strong growth, driven by the rising prevalence of neurovascular diseases such as stroke, brain aneurysms, and arteriovenous malformations, along with increased awareness of minimally invasive treatment options. The demand for early diagnosis and timely intervention has significantly accelerated the adoption of neuro-interventional procedures across hospitals and specialty clinics.
- Technological advancements—such as real-time 3D imaging, flow diversion systems, robotic-assisted navigation, and AI-powered procedural planning—are revolutionizing interventional neurology. These innovations are enhancing the precision, safety, and outcomes of complex neurological interventions, while also streamlining workflow in neuro-endovascular procedures.
- Germany leads the Interventional Neurology market in Europe, capturing the largest revenue share of 26.4% in 2025, accounting for the largest revenue share in 2025, backed by a well-established healthcare infrastructure, early adoption of advanced neurovascular devices, and a high volume of neurointerventional procedures. The country’s leading neurology centers, focus on clinical research, and government-supported stroke care programs further solidify its market leadership.
- Germany is also projected to be the fastest-growing country in the European interventional neurology space over the forecast period. This growth is attributed to an aging population, increasing incidence of ischemic strokes, and expanding reimbursement for mechanical thrombectomy and embolization procedures. Collaborations between medical device companies and academic institutions are accelerating device innovation and clinical adoption.
- Neurothrombectomy Devices are expected to hold the largest market share of 34.5% in 2025 across Europe, driven by their critical role in the treatment of acute ischemic stroke through minimally invasive clot retrieval. The increasing prevalence of stroke, rising awareness about timely intervention, and advancements in device technology such as improved catheter design and enhanced clot retrieval mechanisms contribute to the strong demand for neurothrombectomy systems. These devices offer high success rates in restoring cerebral blood flow, making them indispensable in comprehensive stroke care centers and expanding rapidly in emerging hospitals focused on neurointerventional therapies.
Report Scope and Interventional Neurology Market Segmentation
|
Attributes |
Interventional Neurology Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Interventional Neurology Market Trends
“Advancements in Minimally Invasive Techniques, AI Integration, and Expanded Stroke Care”
- A major trend in the Europe Interventional Neurology market is the increasing adoption of minimally invasive neurovascular procedures, such as mechanical thrombectomy and aneurysm coiling, which reduce patient recovery time and improve outcomes in stroke and vascular disorder management.
- For instance, Medtronic’s Solitaire device has enhanced mechanical thrombectomy efficacy with faster clot retrieval and improved patient prognosis in ischemic stroke cases.
- Artificial intelligence and advanced imaging analytics are being integrated with interventional systems to assist clinicians in real-time decision-making, precise navigation, and personalized treatment planning, leading to improved procedural success and reduced complications.
- There is growing demand for compact, user-friendly devices suitable for emergency and point-of-care use, enabling timely intervention in acute neurovascular events across diverse clinical settings.
- Enhanced connectivity between interventional devices, imaging platforms, and hospital information systems facilitates seamless data exchange, remote procedure monitoring, and collaborative treatment approaches.
- The rising incidence of stroke, neurovascular diseases, and aging populations in Europe is driving the demand for early intervention devices and comprehensive stroke care solutions, reinforcing the critical role of interventional neurology in reducing morbidity and mortality.
Interventional Neurology Market Dynamics
Driver
“Growing Demand for Real-Time, Accurate, and Minimally Invasive Patient Monitoring”
- The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and respiratory conditions in Europe is driving the demand for disposable medical sensors that enable continuous, real-time physiological monitoring with minimal patient discomfort.
- For instance, wearable biosensors for glucose monitoring and cardiac health have become essential for managing chronic illnesses and preventing complications.
- Rising awareness among healthcare providers and patients about the benefits of early detection and remote monitoring is boosting the adoption of disposable sensor technologies.
- Government initiatives and reimbursement policies supporting telehealth and homecare are fueling investments in disposable sensor devices to improve patient outcomes and reduce hospital visits.
- Advances in sensor miniaturization, wireless connectivity, and biocompatible materials enhance the usability and accuracy of disposable sensors, expanding their applications in outpatient and home care settings.
- Growth in point-of-care diagnostics and decentralized healthcare delivery models is accelerating demand for compact, single-use sensors that facilitate rapid, convenient health assessments outside traditional clinical environments.
Restraint/Challenge
“High Costs and Regulatory Barriers”
- The high cost of advanced disposable sensor technologies, especially those with integrated AI, wireless connectivity, and biocompatible materials, limits accessibility for smaller healthcare providers and low-resource settings.
- For instance, single-use biosensors with real-time data transmission capabilities often have price points that challenge adoption in rural clinics and emerging markets.
- Stringent European regulatory requirements, such as CE marking and compliance with the Medical Device Regulation (MDR), extend product approval timelines and increase costs related to testing, documentation, and post-market surveillance.
- A shortage of trained healthcare professionals skilled in deploying and interpreting data from advanced disposable sensors restricts market penetration in less developed areas.
- Variability in sensor accuracy and reliability among manufacturers creates hesitation among clinicians, impacting confidence and widespread use.
- Data privacy and cybersecurity concerns—heightened by integration of disposable sensors with digital health platforms and compliance with GDPR—pose challenges in seamless adoption and trust among users.
Interventional Neurology Market Scope
The market is segmented on the basis product, application, and end user.
- By Product
On the basis of Product, the Interventional Neurology Market is Cerebral Balloon Angioplasty and Stenting Systems, Aneurysm Coiling and Embolization Devices, Neurothrombectomy Devices, and Support Devices. The Cerebral Balloon Angioplasty and Stenting Systems segment is expected to dominate the market with the largest revenue share of 29.7% 2025, dominate due to their widespread use in treating arterial stenosis and preventing ischemic strokes. These minimally invasive devices help restore blood flow in narrowed cerebral arteries. Aneurysm Coiling and Embolization Devices are gaining traction for effective treatment of brain aneurysms, reducing rupture risk.
The Neurothrombectomy Devices segment is projected to witness the fastest CAGR from 2025 to 2032, used for clot removal in ischemic stroke, and Support Devices, such as catheters and guidewires, complete the product portfolio supporting diverse neurointerventional procedures.
- By Application
On the basis of Types, the Interventional Neurology Market is into Vein Stenosis, Artery Stenosis, Brain Aneurysm, Ischemic Strokes, and Others. The Artery Stenosis segment is expected to dominate the market with the largest revenue share, driven by the high incidence of cerebrovascular narrowing that impedes blood flow to the brain, causing strokes and neurological deficits. Brain Aneurysm treatment is expanding due to improved detection and less invasive coiling techniques.
The Ischemic Strokes segment is projected to witness the fastest CAGR from 2025 to 2032, increasingly managed with neurothrombectomy devices to rapidly restore circulation. Vein Stenosis and other neurovascular disorders also contribute to market growth as awareness and diagnostic capabilities improve, enabling timely and targeted interventions.
- By End users
On the basis of end users, the Interventional Neurology market is segmented into Hospitals, Ambulatory Centres, Neurology Clinics, and Others. Hospitals are expected to dominate with the largest revenue share in 2025, as primary end users, offering comprehensive neurointerventional services supported by advanced imaging and specialized care teams. These facilities handle complex cases requiring sophisticated devices and multidisciplinary management.
The Ambulatory Centres segment is projected to witness the fastest growing as outpatient neurovascular interventions become more common, offering cost-effective and convenient care.
Interventional Neurology Market Regional Analysis
- Germany dominates the Europe Interventional Neurology Market, accounting for the largest revenue share of 26.4% in 2025. This dominance stems from its advanced healthcare infrastructure, well-established neurovascular centers, and strong presence of leading medical device manufacturers specializing in cerebral balloon angioplasty, aneurysm coiling, and neurothrombectomy devices.
- Cities like Berlin, Munich, and Frankfurt host world-class hospitals equipped with state-of-the-art imaging and interventional suites. Government initiatives supporting stroke care networks, increasing prevalence of cerebrovascular diseases, and collaborations between device companies and research institutions foster continuous innovation and market growth.
France Interventional Neurology Market Insight
The France Interventional Neurology market is expected to grow steadily, driven by enhanced stroke care programs and expanding neurointerventional capabilities in major cities such as Paris, Lyon, and Marseille. Hospitals and specialized neurology centers are increasingly adopting advanced devices for aneurysm embolization, artery and vein stenosis treatment, and ischemic stroke management. National healthcare policies promoting minimally invasive procedures and reimbursement incentives support broader adoption. Additionally, rising public awareness of stroke symptoms and advancements in diagnostic imaging improve early intervention rates, further fueling market expansion.
U.K. Interventional Neurology Market Insight
The U.K. market for interventional neurology is projected for robust growth, fueled by increased NHS investment in stroke units and neurovascular intervention infrastructure. Leading medical centers in London, Manchester, and Edinburgh are implementing cutting-edge neurothrombectomy systems, stenting devices, and embolization technologies. Despite regulatory adjustments post-Brexit, the U.K. maintains stringent standards for medical device approval, facilitating the introduction of innovative interventional products. The growing focus on rapid stroke intervention, personalized neurovascular care, and expansion of outpatient neurointerventional services drives the market. Rising demand for minimally invasive treatments in emergency and ambulatory settings creates new growth opportunities.
Interventional Neurology Market Share
The Interventional Neurology industry is primarily led by well-established companies, including:
- Medtronic plc (Ireland)
- Stryker Corporation (U.S.)
- Johnson & Johnson (Cordis) (U.S.)
- Terumo Corporation (Japan)
- Penumbra, Inc. (U.S.)
- Boston Scientific Corporation (U.S.)
- MicroVention, Inc. (U.S.)
- Phenox GmbH (Germany)
- Balt Extrusion (France)
- Siemens Healthineers AG (Germany)
Latest Developments in Europe Interventional Neurology Market
- In March 2025, Medtronic plc announced the European launch of its next-generation Pipeline Vantage Embolization Device with Shield Technology, offering improved flow diversion for the treatment of complex brain aneurysms. The product emphasizes enhanced deliverability and reduced thrombogenicity, aligning with the market’s shift toward safer and more effective neurovascular devices.
- In November 2024, Stryker Corporation introduced its Trellis Neurothrombectomy System in select European countries, designed for rapid clot retrieval in ischemic stroke patients. The device integrates aspiration and mechanical thrombectomy, supporting faster recanalization and improved clinical outcomes.
- In August 2024, Penumbra, Inc. received CE mark approval for its RED Reperfusion Catheters in Europe, enabling broader access to advanced stroke intervention tools. The product's enhanced trackability and aspiration efficiency reflect increasing demand for precise and minimally invasive stroke therapies.
- In May 2024, MicroVention, Inc. (a Terumo company) expanded its SOFIA Flow Plus Catheter portfolio in Europe, enabling better access to distal neurovascular anatomy in stroke procedures. This development underscores the trend of device miniaturization and navigability in complex neurovascular interventions.
- In January 2024, Balt Group launched the Silk Vista Baby Flow Diverter across major European neurointerventional centers for the treatment of small and distal intracranial aneurysms. Its approval supports the growing need for specialized devices in treating anatomically challenging cerebrovascular conditions.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.