Europe Medical Device Sterilization Market
Market Size in USD Billion
CAGR :
% 
 USD
1.47 Billion
USD
2.74 Billion
2024
2032
USD
1.47 Billion
USD
2.74 Billion
2024
2032
| 2025 –2032 | |
| USD 1.47 Billion | |
| USD 2.74 Billion | |
|
|
|
|
Europe Medical Device Sterilization Market Size
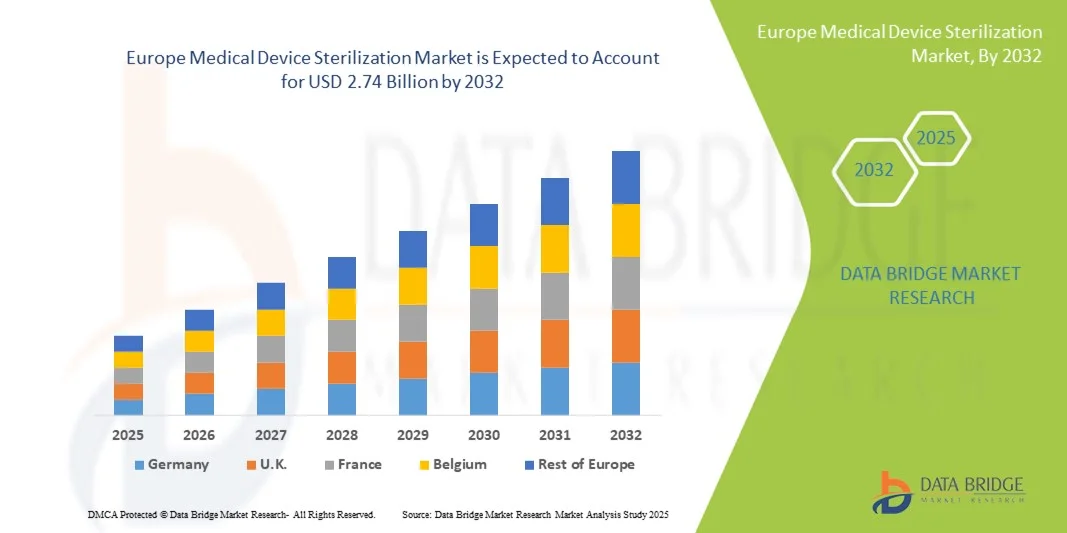
- The Europe medical device sterilization market size was valued at USD 1.47 billion in 2024 and is expected to reach USD 2.74 billion by 2032, at a CAGR of 8.10% during the forecast period
- The market growth is largely driven by the increasing adoption of advanced sterilization technologies, such as ethylene oxide, hydrogen peroxide, and gamma irradiation, across hospitals and surgical centers, ensuring enhanced patient safety and compliance with stringent regulatory standards
- Furthermore, rising healthcare expenditure, growing prevalence of chronic and infectious diseases, and increasing demand for sterile medical devices in both hospital and outpatient settings are accelerating the uptake of Europe Medical Device Sterilization solutions, thereby significantly boosting the industry's growth
Europe Medical Device Sterilization Market Analysis
- Medical device sterilization, encompassing instruments, reagents, and services, is increasingly critical in Europe to ensure patient safety, regulatory compliance, and infection control across hospitals, clinics, laboratories, and pharmaceutical companies
- The growing market demand is primarily driven by rising surgical procedures, increasing prevalence of chronic and infectious diseases, and stringent sterilization regulations in European countries, which mandate high standards of hygiene and safety
- Germany dominated the Europe medical device sterilization market with the largest revenue share of 35.6% in 2024, supported by advanced healthcare infrastructure, high adoption of automated sterilization technologies, and a strong presence of key market players focusing on thermal and gas sterilization solutions
- France is expected to be the fastest-growing country in the market during the forecast period due to expanding healthcare facilities, growing pharmaceutical and medical device manufacturing, and increasing investments in modern sterilization equipment
- Gas and chemical sterilization dominated the market in 2024 with a share of 42.5%, driven by its effectiveness across complex instruments and regulatory acceptance for critical and semi-critical devices
Report Scope and Europe Medical Device Sterilization Market Segmentation
|
Attributes |
Europe Medical Device Sterilization Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Medical Device Sterilization Market Trends
Advancements in Automated and Low-Temperature Sterilization Technologies
- A significant and accelerating trend in the Europe medical device sterilization market is the adoption of automated sterilization systems and low-temperature techniques such as hydrogen peroxide plasma and ethylene oxide, improving safety and efficiency in hospitals and laboratories
- For instance, Steris V-PRO® systems integrate low-temperature sterilization with advanced monitoring, allowing healthcare facilities to sterilize heat-sensitive instruments quickly and safely
- Automation in sterilization equipment enables features such as cycle monitoring, reporting, and error detection, ensuring higher compliance with regulatory standards. For instance, Getinge and Belimed systems provide automated logs and alerts for maintenance and usage deviations
- The integration of advanced sterilization technologies with hospital information systems facilitates centralized tracking of instrument sterilization status, reducing human error and increasing operational efficiency
- This trend towards more precise, monitored, and automated sterilization systems is fundamentally reshaping hospital workflows and compliance expectations. Consequently, companies such as Tuttnauer are developing solutions with automated tracking, low-temperature sterilization options, and connectivity for digital recordkeeping
- The demand for advanced, automated sterilization solutions is growing rapidly across both hospital and pharmaceutical sectors, as healthcare facilities increasingly prioritize patient safety, regulatory compliance, and operational efficiency
Europe Medical Device Sterilization Market Dynamics
Driver
Rising Healthcare Demand and Regulatory Requirements
- The increasing prevalence of chronic and infectious diseases, coupled with stricter sterilization regulations across Europe, is a significant driver for the heightened adoption of medical device sterilization solutions
- For instance, in March 2024, Belimed announced the launch of new automated sterilizers compliant with EU MDR regulations, aiming to enhance patient safety and operational efficiency
- As hospitals and clinics expand their surgical and diagnostic procedures, sterilization solutions provide a critical role in infection control and quality assurance
- Furthermore, the growing production of medical devices and pharmaceuticals in Europe is increasing the demand for advanced sterilization services, ensuring safe and compliant products
- Adoption is also driven by the integration of sterilization equipment with hospital management systems, offering real-time monitoring, reporting, and optimization of sterilization cycles
- Increasing awareness and training programs for infection prevention among healthcare professionals are promoting adoption of advanced sterilization solutions. For instance, workshops in Germany and France demonstrate the benefits of automated systems in reducing infection risk
- Technological collaborations between sterilization equipment manufacturers and hospitals are driving innovation, enabling more efficient and faster sterilization cycles. For instance, Getinge collaborates with major German hospitals to optimize sterilization protocols
Restraint/Challenge
High Equipment Costs and Compliance Complexity
- The relatively high cost of advanced sterilization equipment and consumables poses a significant challenge to broader market penetration, particularly for smaller hospitals or clinics
- For instance, high-cost systems from Getinge and Steris may be less accessible for budget-constrained healthcare facilities, limiting adoption despite their operational benefits
- Regulatory compliance complexity across European countries, including EU MDR and local sterilization standards, increases operational and administrative burdens on end-users
- In addition, some low-temperature sterilization methods require specialized training and maintenance, which can be resource-intensive, posing further barriers to adoption
- Overcoming these challenges through cost-optimization, standardized training programs, and support for regulatory compliance will be vital for sustained market growth across Europe
- Variability in maintenance and service infrastructure across countries can affect the reliability of sterilization equipment and discourage investment in advanced systems. For instance, smaller clinics in Eastern Europe may lack technical support for complex systems
- Limited awareness among some end-users regarding the long-term cost savings and efficiency of automated sterilization solutions can hinder adoption. For instance, some hospitals in France still rely heavily on manual sterilization despite the operational benefits of automation
Europe Medical Device Sterilization Market Scope
The market is segmented on the basis of product, technology, end-user, and distribution channel.
- By Product
On the basis of product, the Europe medical device sterilization market is segmented into instruments, reagents, and services. The instruments segment dominated the market with the largest revenue share in 2024, driven by the widespread use of sterilizable surgical tools, diagnostic instruments, and critical medical devices across hospitals and clinics. Instruments require stringent sterilization protocols to ensure patient safety, compliance with EU MDR regulations, and prevention of hospital-acquired infections. Hospitals and surgical centers prioritize instrument sterilization due to the direct impact on surgical outcomes and infection control standards. Advanced sterilization methods, such as low-temperature hydrogen peroxide plasma, are increasingly applied to instruments to maintain functionality while ensuring sterility. The high cost and long lifecycle of instruments further necessitate regular sterilization, boosting demand for these solutions. For instance, Getinge and Tuttnauer provide instrument-specific sterilization cycles with integrated monitoring for healthcare facilities.
The services segment is anticipated to witness the fastest growth from 2025 to 2032, driven by outsourcing of sterilization processes by smaller hospitals, clinics, and medical device manufacturers. Services offer cost efficiency, compliance assurance, and access to advanced sterilization technologies without the need for in-house capital investment. Pharmaceutical companies increasingly rely on third-party sterilization services for reagents and critical devices. Service providers often provide tailored solutions, including cycle monitoring, reporting, and validation, which appeal to institutions aiming to reduce operational burden. The growth is further fueled by the expansion of specialized sterilization centers across Europe, offering fast turnaround and regulatory-compliant sterilization.
- By Technology
On the basis of technology, the Europe medical device sterilization market is segmented into thermal sterilization, ionizing radiation sterilization, filtration sterilization, and gas and chemical sterilization. The gas and chemical sterilization segment dominated the market with the largest revenue share of 42.5% in 2024, attributed to its proven effectiveness in sterilizing heat- and moisture-sensitive medical devices. Gas sterilization methods, such as ethylene oxide and hydrogen peroxide plasma, are widely used across hospitals, laboratories, and pharmaceutical manufacturing. The segment is preferred for its regulatory acceptance for critical and semi-critical instruments and the ability to preserve instrument integrity. Hospitals with high volumes of complex surgical instruments rely heavily on gas sterilization to meet infection control standards. In addition, major manufacturers such as Steris and Belimed provide fully automated gas sterilization systems with monitoring and reporting features. For instance, V-PRO® sterilizers enable low-temperature chemical sterilization suitable for delicate medical instruments.
The ionizing radiation sterilization segment is expected to witness the fastest growth from 2025 to 2032, driven by its increasing adoption in pharmaceutical manufacturing and single-use medical devices. Radiation sterilization, including gamma and electron beam methods, offers high throughput and precise sterilization without residual chemicals. Growing production of disposable syringes, catheters, and diagnostic kits is accelerating the demand for radiation sterilization services. The method’s efficiency, scalability, and ability to maintain product quality make it attractive for manufacturers. Companies such as Nordion and Synergy Health are expanding capacity to meet rising demand across Europe.
- By End-User
On the basis of end-user, the market is segmented into pharmaceutical companies, hospitals, clinics, laboratories, academic and research institutes, medical device manufacturers, and others. The hospitals segment dominated the Europe medical device sterilization market in 2024, driven by the high volume of surgical procedures, diagnostics, and infection-sensitive treatments requiring sterilized instruments and consumables. Hospitals prioritize automated and validated sterilization cycles to reduce infection risks and comply with EU MDR and national healthcare regulations. Both public and private hospitals invest in advanced sterilizers to improve operational efficiency and patient safety. The demand is particularly strong in tertiary and quaternary care hospitals with specialized surgical departments. For instance, German and French hospitals widely adopt integrated sterilization systems that track instrument usage and compliance.
The pharmaceutical companies segment is expected to witness the fastest growth from 2025 to 2032 due to increasing production of sterile drugs, biologics, and injectable formulations. Pharmaceutical manufacturers require precise sterilization of instruments, vials, and reagents to meet stringent regulatory standards and ensure product safety. The growth is further supported by the rising contract manufacturing and outsourcing of sterilization processes. Services that offer validated sterilization, real-time monitoring, and reporting are in high demand for compliance and efficiency. Companies such as Sartorius and Steris are expanding offerings tailored to pharmaceutical sterilization requirements across Europe.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into direct tenders, retail sales, and third-party distributors. The direct tenders segment dominated the market in 2024, driven by procurement practices in hospitals, clinics, and pharmaceutical companies that favor direct purchases from manufacturers. Direct tenders ensure compliance with EU regulations, allow access to after-sales service, and enable customization of sterilization solutions to meet specific end-user needs. Hospitals and large clinics prefer this channel for high-value equipment such as automated sterilizers, chemical sterilization units, and instrument-specific solutions. For instance, Getinge and Belimed supply sterilization systems directly to leading German and French hospitals.
The third-party distributor segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing outsourcing of sterilization consumables, reagents, and small-scale equipment. Distributors provide flexibility, localized support, and quicker delivery times for smaller hospitals, clinics, and research institutes. Growth in the third-party channel is further supported by expanding presence of specialized distributors across Europe, catering to multiple end-users with bundled services and regulatory support. For instance, Tuttnauer and Steris collaborate with distributors to reach smaller healthcare facilities in Eastern Europe efficiently.
Europe Medical Device Sterilization Market Regional Analysis
- Germany dominated the Europe medical device sterilization market with the largest revenue share of 35.6% in 2024, supported by advanced healthcare infrastructure, high adoption of automated sterilization technologies, and a strong presence of key market players focusing on thermal and gas sterilization solutions
- Healthcare providers in the region highly value the reliability, advanced sterilization technologies, and compliance with international safety standards offered by Germany-based sterilization solutions
- This widespread adoption is further supported by continuous technological innovation, increasing healthcare expenditures, and a focus on infection control, establishing medical device sterilization as a critical solution for both hospitals and medical device manufacturers across Europe
The U.K. Medical Device Sterilization Market Insight
The U.K. medical device sterilization market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing surgical volumes and strict regulatory standards. Concerns regarding infection control are encouraging hospitals and clinics to adopt modern sterilization solutions. The U.K.’s robust healthcare infrastructure, adoption of advanced sterilization technologies, and emphasis on patient safety are expected to continue stimulating market growth.
Germany Medical Device Sterilization Market Insight
The Germany medical device sterilization market is expected to expand at a considerable CAGR during the forecast period, fueled by rising demand from hospitals, clinics, and medical device manufacturers. Germany’s well-developed healthcare infrastructure, strong medical device industry, emphasis on innovation, and stringent regulatory environment promote adoption of advanced sterilization technologies. Integration of sterilization systems into hospital workflows, along with preferences for reliable, sustainable, and high-efficiency solutions, is increasingly prevalent.
France Medical Device Sterilization Market Insight
The France medical device sterilization market is poised for steady growth, driven by increasing surgical procedures, modernizing hospital infrastructure, and stringent hygiene regulations. Healthcare providers are adopting advanced sterilization technologies to ensure compliance with national and EU standards. The growing focus on infection prevention and patient safety, combined with technological advancements in sterilization equipment, is fueling market expansion in both hospitals and medical device manufacturing facilities.
Poland Medical Device Sterilization Market Insight
The Poland medical device sterilization market is expected to grow at a notable CAGR during the forecast period, fueled by rising investments in healthcare infrastructure and modernization of hospitals. Adoption of advanced sterilization solutions is increasing due to stricter regulatory requirements and the growing number of medical procedures. Poland’s healthcare sector is increasingly focusing on infection control and patient safety, driving demand for reliable and efficient sterilization technologies in both clinical and manufacturing settings.
Europe Medical Device Sterilization Market Share
The Europe Medical Device Sterilization industry is primarily led by well-established companies, including:
- Steritech (U.S.)
- BGS Beta-Gamma-Service GmbH & Co. KG (Germany)
- DE LAMA S.P.A. (Italy)
- Andersen Products Ltd (U.K.)
- Steriflow (France)
- Sterimed (France)
- SysTec Systemtechnik und Industrieautomation GmbH (Germany)
- Noxilizer, Inc. (U.S.)
- SHP Steriltechnik AG (Germany)
- Matachana Group (Spain)
- Sterigenics International LLC (U.S.)
- Alvimedica (Turkey)
- Getinge AB (Sweden)
- Medisafe International Ltd (U.K.)
- STERIS (Ireland)
- Lohmann & Rauscher GmbH & Co. KG (Germany)
- Belimed AG (Switzerland)
- Metall Zug AG (Switzerland)
What are the Recent Developments in Europe Medical Device Sterilization Market?
- In June 2025, The European Commission announced a regulation allowing healthcare professionals to receive instructions for medical devices in electronic format, rather than solely on paper. This move aims to further digitalize healthcare systems and reduce paper waste
- In June 2025, The European Union decided to bar Chinese companies from most public tenders for medical devices valued at over five million euros. This measure is part of the EU's International Procurement Instrument, aiming to ensure reciprocal market access and address unbalanced procurement practices
- In April 2025, The European Commission introduced updated standards for medical device sterilization, aiming to improve safety and efficacy. These revisions focus on harmonizing sterilization processes across member states, ensuring that medical devices meet stringent sterilization requirements before reaching the market
- In March 2025, The FDA acknowledged the revised ANSI/AAMI ST58 standard, which includes information on new technologies and chemical sterilization modalities, including ethylene oxide. This update reflects advancements in sterilization methods and regulatory practices
- In January 2025, SGS, a leading inspection, verification, testing, and certification company, announced the extension of its sterilization services under the EU Medical Device Regulation (MDR) 2017/745. The Federal Agency for Medicines and Health Products (FAMHP) approved SGS's expanded scope after verifying its training, qualifications, and in-house competence
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.