Europe Menopausal Disorder Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.30 Billion
USD
5.46 Billion
2022
2030
USD
3.30 Billion
USD
5.46 Billion
2022
2030
| 2023 –2030 | |
| USD 3.30 Billion | |
| USD 5.46 Billion | |
|
|
|
|
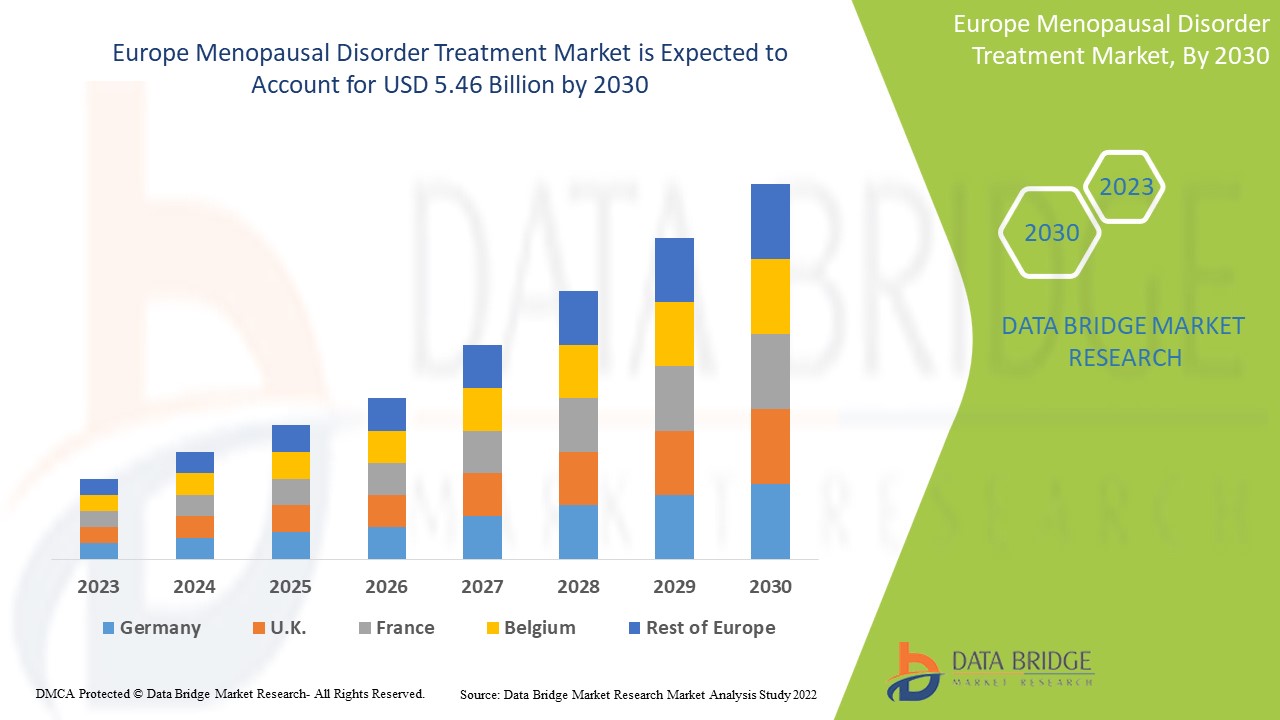
Europe Menopausal disorder Treatment Market Analysis and Size
In the recent years, the market is estimated to grow rapidly during the forecast period. Menopause is one of the most physically demanding times in any women’s life, encouraging pharmaceutical companies globally to create effective medications to treat it and its associated problems. Menopausal problems are becoming more prevalent in all parts of the world, and several healthcare organizations support developing and using medications that can effectively treat them.
Data Bridge Market Research analyses a growth rate in the menopausal disorder treatment market in the forecast period 2023-2030. The expected CAGR of menopausal disorder treatment market is tend to be around 6.5% in the mentioned forecast period. The market was valued at USD 3.3 billion in 2022, and it would grow upto USD 5.46 billion by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Menopausal disorder Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Stages (Menopause, Postmenopause, Perimenopause), Type (Hormonal Therapy and Non-Hormonal Therapy), Menopause Symptoms (Vasomotor Symptoms, Atrophic Vaginitis, Osteoporosis, Joint Pain, Weight Gain, Dyspareunia, Depression, Others), Dosage Form (Tablet, Cream, Gels, Rings/Insert, Patch/Film, Injection Shot, Spray, Others), Route of Administration (Oral, Vaginal, Transdermal, Parenteral), End User (Hospital/Clinical Laboratories, Physician Offices, Reference Laboratories and Other End Users), Distribution Channel (Direct Tender, Retail Sales and Other) |
|
Countries Covered |
U.K., Germany, France, Spain, Italy, Netherlands, Switzerland, Russia, Turkey, Belgium |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Sanofi (France), Pfizer Inc. (U.S.), GSK plc (U.K.), Novartis AG (Switzerland), AstraZeneca (U.K.), Johnson & Johnson Private Limited (U.S.), Merck & Co., Inc. (U.S.), Eli Lilly and Company (U.S.), Abbott (U.S.), Novo Nordisk A/S (Denmark), Abbvie,Inc (U.S.), Dr. Reddy’s Laboratories Ltd. (India), TherapeuticsMD, Inc. (U.S.), Ipsen Pharma (France), Besins Healthcare (Belgium), Mithra Pharmaceuticals (Belgium), Aché Laboratórios Farmacêuticos S.A (Brazil), SEBELA PHARMACEUTICALS (U.S.), Fervent Pharmaceuticals Inc. (U.S.), KaNDy Therapeutics (U.K.), Duchesnay (U.S.) |
|
Market Opportunities |
|
Market Definition
Menopause is a natural biological process that occurs because of the reduction of reproductive hormones. This marks the end of the menstrual cycle. It generally occurs in the age of late 40s or 50s. The menopause symptoms are different for every women. Every woman experiences these menopause symptoms in a different way. Estrogen levels decline throughout menopause. These signs and symptoms include night sweats, vaginal dryness, hot flashes, abnormal menstrual cycles, difficulty sleeping, mood swings, and others.
Menopausal Disorder Treatment Market Dynamics
Drivers
- Increased Drug Development Processes
A rise in the number of menopausal drugs in the drug development pipeline of numerous key manufacturers is one of the biggest market growth opportunities. It includes specific and innovative drugs, that focus on decreasing side effects and providing improved efficacy. For instance, Fuji Pharma Co., Ltd. has a natural progesterone drug (FSN-011-01) in its pipeline, and has submitted an application for marketing approval of the same to the Ministry of Health, Labour and Welfare, Japan.
- Higher Demand of Herbal Treatments
With each passing decade, women are drifting more towards herbal supplements as a menopause treatment which is one of the market's most prominent latest developments. As a result, several companies are responding to the market need by manufacturing new products which contain menopause-relieving components. For instance, black cohosh and soy isoflavones are the two natural remedies that worked well for menopause.
Opportunities
- Major Impact of Trending Lifestyle and Stress
In recent years, it has become favourably important to provide women with much effective therapy for menopause symptoms due to the rise in the incidence of physical and mental health issues among women. Factors such as hot flashes, migraine, anxiety, formication, fatigue, mood swings, irritability, and insomnia, contribute a lot to many companies to develop cutting-edge therapies to improve these symptoms. Immense growth in the menopause therapy industry is being supported by extensive investments in extended R&D initiatives to launch novel medications.
- Rising Demand of Oral Drugs
Oral drugs are one of the easiest, most durable, most affordable, and portable. It has been seen that the rate of contamination is also the least. This makes this route of administration more beneficial. The oral mode of administration is found to be the most accessible, cost-effective, and extensively used mode of medication administration.
Restraints/Challenges
- Increasing Underlying Disparities Between Genders
Several companies providing multiple treatments for menopause have struggled significantly due to the widespread existence of major gender-wise health disparities in many of the nations. Women in nations with restricted awareness and income see no gains in their health because of the lack of adequate healthcare infrastructure. This acts as a major restriction for the market.
- Side Effects of Hormone Therapy
As per the FDA, various complications associated with hormone therapy carry numerous hazards, including the possibility of stroke, heart problems, blood clots, and breast cancer. Thus, it hampers the market growth.
This menopausal disorder treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the menopausal disorder treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Europe Menopausal disorder Treatment Market
The menopausal disorder treatment market is segmented on the basis of stages, type, menopause symptoms, dosage form, route of administration, end-user and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Stages
- Menopause
- Postmenopause
- Perimenopause
Type
- Hormonal Therapy
- Non-Hormonal Therapy
Menopause Symptoms
- Vasomotor Symptoms
- Atrophic Vaginitis
- Osteoporosis
- Joint Pain
- Weight Gain
- Dyspareunia
- Depression
- Others
Dosage Form
- Tablet
- Cream
- Gels
- Rings/Insert
- Patch/Film
- Injection Shot
- Spray
- Others
Route of Administration
- Oral
- Vaginal
- Transdermal
- Parenteral
End User
- Hospital/Clinical Laboratories
- Physician Offices
- Reference Laboratories
- Others
Distribution Channel
- Direct Tender
- Retail Sales
- Other
Europe Menopausal Disorder Treatment Market Regional Analysis/Insights
The menopausal disorder treatment market is analyzed and market size insights and trends are provided by stages, type, menopause symptoms, dosage form, route of administration, end-user and distribution channel as referenced above.
The major countries covered in the menopausal disorder treatment market report are U.K., Germany, France, Spain, Italy, Netherlands, Switzerland, Russia, Turkey, Belgium .
Europe dominates the menopausal disorder treatment market because of rising introduction of novel drugs by several key players.
Germany is dominating the menopausal disorder treatment market due to the growing demand of many effective treatments such as hormone replacement therapy.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Menopausal Disorder Treatment Market Share Analysis
The menopausal disorder treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to menopausal disorder treatment market
Key players operating in the menopausal disorder treatment market t include:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France), Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- Johnson & Johnson Private Limited (U.S.)
- Merck & Co., Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Abbott (U.S.)
- Novo Nordisk A/S (Denmark)
- Abbvie, Inc (U.S.)
- Dr. Reddy’s Laboratories Ltd. (India)
- TherapeuticsMD, Inc. (U.S.)
- Ipsen Pharma (France)
- Besins Healthcare (Belgium)
- Mithra Pharmaceuticals (Belgium)
- Aché Laboratórios Farmacêuticos S.A (Brazil)
- SEBELA PHARMACEUTICALS (U.S.)
- Fervent Pharmaceuticals Inc. (U.S.)
- KaNDy Therapeutics (U.K.)
- Duchesnay (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE MENOPAUSAL DISORDER TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE MENOPAUSAL DISORDER TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY STAGES
15.1 OVERVIEW
15.2 PERIMENOPAUSE
15.3 MENOPAUSE
15.4 POSTMENOPAUSE
16 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY TYPE
16.1 OVERVIEW
16.2 HORMONAL THERAPY
16.2.1 ESTROGEN
16.2.1.1. 17Β-ESTRADIOL
16.2.1.1.1. ESTRADERM
16.2.1.1.2. ESTRADOT
16.2.1.1.3. MINIVELLE
16.2.1.1.4. CLIMARA
16.2.1.1.5. ESTRACE
16.2.1.1.6. OTHERS
16.2.1.2. CONJUGATED ESTROGENS
16.2.1.2.1. PREMARIN
16.2.1.2.2. ENJUVIA
16.2.1.2.3. C.E.S. PMS
16.2.1.2.4. OTHERS
16.2.1.3. ESTERIFIED ESTROGENS
16.2.1.3.1. MENEST
16.2.1.3.2. ESTRAGYN
16.2.1.3.3. OTHERS
16.2.1.4. ESTROPIPATE
16.2.1.4.1. HARMOGEN
16.2.1.4.2. IMPROVERA
16.2.1.4.3. OGEN
16.2.1.4.4. OTHERS
16.2.1.5. ESTRADIOL ACETATE
16.2.1.5.1. FEMTRACE
16.2.1.5.2. FEMRING
16.2.1.5.3. MENORING
16.2.1.5.4. OTHERS
16.2.1.6. ESTRADIOL HEMIHYDRATE
16.2.1.6.1. VAGIFEM
16.2.1.6.2. VAGIFEM 10
16.2.1.6.3. OTHERS
16.2.1.7. OTHERS
16.2.2 PROGESTOGENS
16.2.2.1. MEDROXYPROGESTERONE ACETATE
16.2.2.1.1. PROVERA
16.2.2.1.2. OTHERS
16.2.2.2. MICRONIZED PROGESTERONE
16.2.2.2.1. PROMETRIUM
16.2.2.2.2. OTHERS
16.2.3 COMBINED ESTROGEN & PROGESTOGEN DRUGS
16.2.3.1. CONJUGATED ESTROGENS (E) + MEDROXYPROGESTERONE ACETATE (P)
16.2.3.1.1. PREMPHASE
16.2.3.1.2. PREMPRO
16.2.3.1.3. OTHERS
16.2.3.2. 17Β-ESTRADIOL (E) + NORETHINDRONE ACETATE (P
16.2.3.2.1. ACTIVELLE LD
16.2.3.2.2. COMBIPATCH
16.2.3.2.3. ESTALIS
16.2.3.2.4. OTHERS
16.2.3.3. ETHINYL ESTRADIOL (E) + NORETHINDRONE ACETATE (P)
16.2.3.3.1. FEMHRT
16.2.3.3.2. FEMHRT LO
16.2.3.3.3. OTHERS
16.2.3.4. OTHERS
16.2.4 OTHERS
16.3 NON-HORMONAL THERAPY
16.3.1 MEDICATION
16.3.1.1. OSPEMIFENE
16.3.1.1.1. OSPHENA
16.3.1.1.2. OTHERS
16.3.1.2. PAROXETINE
16.3.1.2.1. BRISDELLE
16.3.1.2.2. OTHERS
16.3.1.3. ANTI-DEPRESSANTS
16.3.1.3.1. EFFEXOR
16.3.1.3.2. PROZAC
16.3.1.3.3. ZOLOFT
16.3.1.3.4. OTHERS
16.3.1.4. GABAPENTIN
16.3.1.5. CLONIDINE
16.3.1.6. OTHERS
16.3.2 SUPPLEMENTS
16.3.2.1. HERBAL SUPPLEMENTS
16.3.2.1.1. GINSENG
16.3.2.1.2. ASHWAGANDHA
16.3.2.1.3. OTHERS
16.3.2.2. VITAMIN D
16.3.2.3. OTHERS
16.4 ALTERNATE TREATMENT
16.4.1 YOGA
16.4.2 TAI CHI
16.4.3 HYPNOSIS
16.4.4 COGNITIVE BEHAVIORAL THERAPY
16.4.5 BIOFEEDBACK AND RELAXATION TRAINING
16.4.6 MINDFULNESS-BASED STRESS REDUCTION
16.4.7 AROMATHERAPY
16.4.8 OTHERS
16.5 SURGERY (OOPHORECTOMY)
17 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY BRAND
17.1 OVERVIEW
17.2 BRANDED
17.3 GENERICS
18 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY PRESCRIPTION MODE
18.1 OVERVIEW
18.2 OVER THE COUNTER
18.3 PRESCRIPTION BASED
19 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
19.1 OVERVIEW
19.2 ORAL
19.2.1 TABLET
19.2.2 PILLS
19.2.3 OTHERS
19.3 PARENTERAL/ INJECTION SHOT
19.3.1 INTRAVENOUS
19.3.2 SUBCUTANEOUS
19.3.3 OTHERS
19.4 TRANSDERMAL
19.4.1 GELS
19.4.2 CREAM
19.4.3 PATCH/FILM
19.4.4 SPRAY
19.5 VAGINAL (RINGS)
19.6 OTHERS
20 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY AGE GROUP
20.1 OVERVIEW
20.2 40-50 YEARS
20.3 ABOVE 50 YEARS
21 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITAL
21.2.1 PUBLIC
21.2.2 PRIVATE
21.3 SPECIALTY CLINICS/CLINICAL LABORATORIES
21.4 REFERENCE LABORATORIES
21.5 PHYSICIAN OFFICES
21.6 HOME HEALTHCARE
21.7 OTHER END USERS
22 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAIL SALES
22.3.1 HOSPITAL PHARMACY
22.3.2 ONLINE PHARMACY
22.3.3 RETAIL PHARMACY
22.4 OTHERS
23 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, BY COUNTRY
EUROPE MENOPAUSAL DISORDER TREATMENT MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, SWOT AND DBMR ANALYSIS
26 EUROPE MENOPAUSAL DISORDER TREATMENT MARKET, COMPANY PROFILE
26.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPMENTS
26.2 ABBVIE INC.
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 PFIZER INC.
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPMENTS
26.4 NOVO NORDISK A/S
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 MYLAN N.V. (VIATRIS INC.)
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 NOVARTIS AG
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 ZYDUS LIFESCIENCES LIMITED
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 MITHRA PHARMACEUTICALS
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 AMGEN INC.
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.1 GSK PLC
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 LUPIN PHARMACEUTICALS, INC.
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 GADOR SA
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 QUÍMICA MONTPELLIER SA
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 ABBOTT
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 BAYER SA
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 LABORATORIOS BAGÓ S.A.
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 SEBELA PHARMACEUTICALS INC.
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 ELI LILLY AND COMPANY + ALKERMES, INC.
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 DR. REDDY’S LABORATORIES LTD
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 DUCHESNAY USA
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












