Europe Mobile Cardiac Telemetry Mct Market
Market Size in USD Million
CAGR :
% 
 USD
946.90 Million
USD
2,245.87 Million
2025
2033
USD
946.90 Million
USD
2,245.87 Million
2025
2033
| 2026 –2033 | |
| USD 946.90 Million | |
| USD 2,245.87 Million | |
|
|
|
|
Europe Mobile Cardiac Telemetry (MCT) Market Size
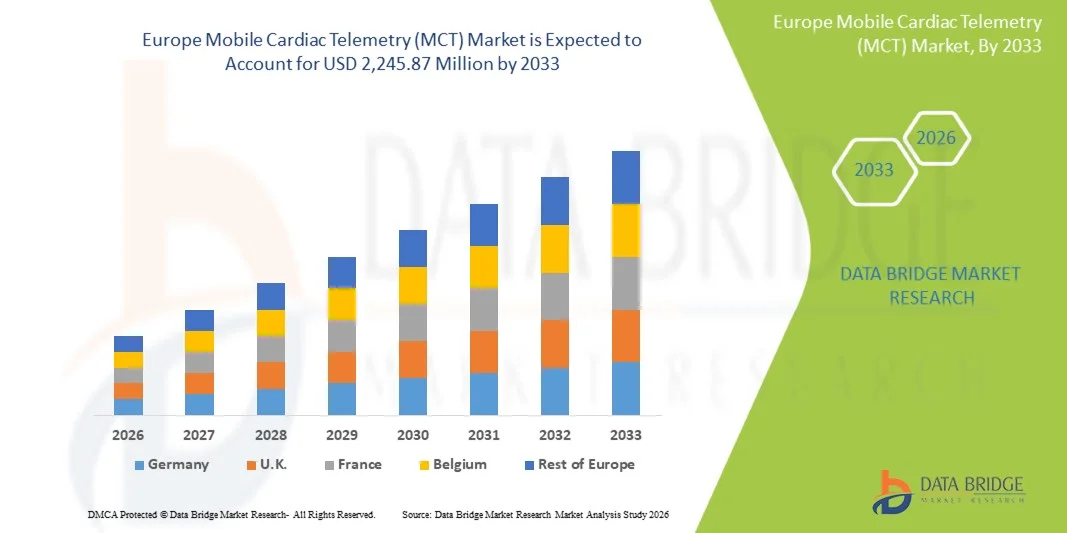
- The Europe Mobile Cardiac Telemetry (MCT) market size was valued at USD 946.90 million in 2025 and is expected to reach USD 2,245.87 million by 2033, at a CAGR of 11.4% during the forecast period
- The market growth is largely fueled by the increasing prevalence of cardiovascular diseases, coupled with advancements in remote patient monitoring and wearable cardiac monitoring technologies, driving the adoption of MCT solutions across healthcare settings
- Furthermore, rising demand for real-time, accurate, and continuous cardiac monitoring, along with healthcare providers’ focus on early diagnosis and improved patient outcomes, is establishing MCT devices as a critical tool in modern cardiac care. These converging factors are accelerating the adoption of MCT solutions, thereby significantly boosting the industry's growth
Europe Mobile Cardiac Telemetry (MCT) Market Analysis
- Mobile Cardiac Telemetry (MCT) devices, providing continuous remote monitoring of cardiac rhythms, are increasingly vital components of modern cardiovascular care in both outpatient and hospital settings due to their real-time monitoring capabilities, early arrhythmia detection, and seamless integration with digital health platforms
- The escalating demand for MCT solutions is primarily fueled by the rising prevalence of cardiovascular diseases, growing awareness of remote patient monitoring, and a preference for continuous, non-invasive cardiac monitoring over traditional intermittent methods
- Germany dominated the Europe MCT market with the largest revenue share of 38.7% in 2025, characterized by advanced healthcare infrastructure, high adoption of digital health technologies, and a strong presence of key medical device players, with substantial growth in MCT adoption driven by innovations in wearable telemetry devices and AI-enabled arrhythmia detection
- France is expected to be the fastest-growing country in the Europe MCT market during the forecast period due to increasing healthcare digitization, expanding reimbursement policies, and rising investments in cardiac care infrastructure
- Patch Based segment dominated the market with a market share of 45.8% in 2025, driven by their patient-friendly design, ease of use, and capability to provide continuous, long-term cardiac monitoring without disrupting daily activities
Report Scope and Europe Mobile Cardiac Telemetry (MCT) Market Segmentation
|
Attributes |
Europe Mobile Cardiac Telemetry (MCT) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Europe Mobile Cardiac Telemetry (MCT) Market Trends
“Advancements in AI-Enabled and Wearable Cardiac Monitoring”
- A significant and accelerating trend in the Europe MCT market is the integration of artificial intelligence (AI) and wearable technologies into cardiac monitoring devices, enhancing real-time arrhythmia detection, predictive analytics, and patient compliance
- For instance, BioTelemetry’s AI-enabled MCT devices utilize machine learning algorithms to detect abnormal cardiac rhythms and predict potential arrhythmic events, providing clinicians with actionable insights for timely intervention
- AI integration in MCT devices allows for personalized monitoring, improved diagnostic accuracy, and reduction of false alerts, while wearable form factors enhance patient comfort and adherence to long-term monitoring protocols
- Wearable MCT devices are increasingly compatible with smartphone apps and digital health platforms, enabling centralized monitoring of cardiac health, data sharing with physicians, and integration with other remote patient monitoring tools
- Integration with cloud-based analytics platforms allows healthcare providers to track patient trends over time, facilitating early intervention and long-term cardiac care planning
- Partnerships between device manufacturers and telehealth service providers are creating comprehensive cardiac monitoring ecosystems that improve patient engagement and remote care efficiency
- This trend towards intelligent, connected, and patient-friendly MCT systems is reshaping expectations for cardiac care, driving demand for continuous, non-invasive, and AI-assisted cardiac monitoring solutions
- The adoption of AI-enabled wearable MCT devices is growing rapidly across hospitals, clinics, and home care settings, as healthcare providers increasingly prioritize early diagnosis, real-time monitoring, and improved patient outcomes
Europe Mobile Cardiac Telemetry (MCT) Market Dynamics
Driver
“Increasing Prevalence of Cardiovascular Diseases and Remote Monitoring Demand”
- The rising incidence of cardiovascular disorders in Europe, coupled with growing awareness of remote patient monitoring, is a key driver for MCT adoption across outpatient and home care settings
- For instance, in March 2025, iRhythm Technologies expanded its European MCT offerings with AI-powered devices to support continuous arrhythmia detection and remote monitoring for high-risk patients
- The increasing need for real-time, accurate, and continuous cardiac data is motivating healthcare providers to deploy MCT solutions for improved diagnostic precision, timely intervention, and reduced hospital readmissions
- Moreover, the growing focus on preventive healthcare, patient-centric monitoring, and telehealth initiatives is making MCT devices integral to modern cardiovascular care strategies
- Features such as remote data transmission, automated alerts for abnormal cardiac events, and cloud-based reporting are enhancing the efficiency of cardiac monitoring, driving adoption in both clinical and home care environments
- Rising reimbursement support from public and private healthcare providers for remote cardiac monitoring solutions is further encouraging hospitals and clinics to implement MCT technologies
- Increasing patient preference for non-invasive, convenient, and continuous monitoring solutions at home is also propelling the growth of the MCT market in Europe
Restraint/Challenge
“High Device Costs and Regulatory Compliance Barriers”
- The high costs associated with advanced MCT devices and subscription-based monitoring services can restrict adoption, especially in price-sensitive European healthcare markets, posing a notable challenge to market growth
- For instance, small clinics or emerging healthcare providers may hesitate to invest in premium AI-enabled MCT solutions due to upfront costs and ongoing monitoring fees
- Regulatory compliance requirements for medical devices, including CE marking and GDPR data privacy regulations, can create hurdles for market entry and device deployment across multiple European countries
- Ensuring secure transmission of patient cardiac data, maintaining device accuracy, and meeting clinical validation standards are critical to overcoming compliance and trust issues among healthcare providers
- In addition, patient adherence challenges, such as discomfort with wearable devices or reluctance to use remote monitoring tools, may limit the effectiveness and widespread adoption of MCT solutions
- Complex integration with existing hospital IT systems can pose operational challenges and slow adoption rates among healthcare providers
- Limited awareness among smaller clinics and patients regarding the benefits of continuous MCT monitoring may restrict market penetration in certain European regions
- Addressing cost barriers, ensuring regulatory compliance, and enhancing patient engagement through user-friendly wearable designs and integrated digital platforms will be vital for sustained market growth
Europe Mobile Cardiac Telemetry (MCT) Market Scope
The market is segmented on the basis of technology, type, cellular connection, and end user.
- By Technology
On the basis of technology, the Europe MCT market is segmented into lead-based and patch-based devices. The patch-based segment dominated the market with the largest revenue share of 45.8% in 2025, driven by its non-invasive, wearable design that enhances patient comfort and long-term adherence. Patch-based devices allow continuous cardiac monitoring without restricting daily activities, making them highly preferred in both home healthcare and hospital settings. Their compatibility with smartphone apps and cloud-based platforms enables real-time data transmission and remote monitoring, further increasing their adoption. Healthcare providers favor patch-based MCT solutions for early detection of arrhythmias and reduction in hospital readmissions. The ease of application and maintenance, coupled with AI-assisted data analysis, makes patch-based devices a standard choice in modern cardiac care.
The lead-based segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by rising adoption in clinical diagnostics and specialized cardiac centers. Lead-based MCT devices provide multi-lead recordings with higher diagnostic accuracy, which is critical for complex arrhythmia cases. Hospitals and ambulatory surgical centers prefer lead-based solutions for detailed cardiac evaluations, particularly for high-risk patients requiring precise monitoring. Continuous technological advancements, such as wireless lead-based systems, are enhancing mobility and patient convenience. The combination of clinical accuracy and integration with telehealth platforms is expected to drive the growth of lead-based MCT devices across Europe.
- By Type
On the basis of type, the Europe MCT market is segmented into multi-channel and single-channel devices. The single-channel segment dominated the market in 2025 due to its simplicity, cost-effectiveness, and suitability for long-term home monitoring. Single-channel MCT devices are widely adopted for routine cardiac surveillance and arrhythmia detection in outpatient care. Their lightweight and wearable design improves patient compliance, and their integration with mobile apps allows healthcare providers to receive real-time alerts and automated reports. Single-channel devices are also preferred by independent diagnostic testing facilities (IDTFs) and home healthcare providers due to ease of deployment and minimal maintenance requirements. The combination of affordability and efficiency ensures sustained demand in residential and clinical settings.
The multi-channel segment is expected to witness the fastest CAGR from 2026 to 2033, driven by its ability to provide comprehensive cardiac monitoring for complex cases. Multi-channel devices record multiple ECG leads simultaneously, offering detailed insights into arrhythmia patterns, conduction abnormalities, and ischemic events. Hospitals, cardiac centers, and high-risk patient monitoring programs prefer multi-channel MCT solutions for accurate diagnosis and treatment planning. The integration of AI algorithms with multi-channel devices further enhances predictive capabilities and reduces false alerts. Increasing awareness among cardiologists regarding the diagnostic benefits of multi-channel monitoring is expected to fuel market growth during the forecast period.
- By Cellular Connection
On the basis of cellular connection, the Europe MCT market is segmented into wireless (Wi-Fi) and Bluetooth (BT). The wireless (Wi-Fi) segment held the largest market revenue share in 2025, owing to its ability to transmit real-time cardiac data directly to cloud platforms for continuous remote monitoring. Wi-Fi-enabled MCT devices allow physicians to monitor patients from anywhere, enabling early intervention in case of abnormal cardiac events. Hospitals and home healthcare providers widely prefer Wi-Fi devices for their reliable connectivity and integration with telehealth systems. The availability of Wi-Fi networks across urban and semi-urban areas facilitates seamless deployment and scalability of MCT monitoring programs. Continuous updates, automated reporting, and compatibility with AI analytics contribute to their dominance in the European market.
The Bluetooth segment is expected to witness the fastest CAGR from 2026 to 2033 due to its low power consumption, ease of pairing with smartphones, and direct peer-to-peer communication. Bluetooth-enabled MCT devices are ideal for localized home monitoring and patient mobility, offering flexibility in device placement and usage. Patients using wearable Bluetooth devices can transmit data during consultations or sync periodically with their providers without continuous internet dependence. Growing preference for portable, low-energy cardiac monitoring solutions in outpatient and home healthcare settings is driving the adoption of Bluetooth-connected MCT devices.
- By End User
On the basis of end user, the Europe MCT market is segmented into home healthcare, hospitals, independent diagnostic testing facilities (IDTF), cardiac centers, and ambulatory surgical centers. The hospitals segment dominated the market in 2025, driven by high patient inflow, established cardiac care infrastructure, and the need for continuous arrhythmia monitoring for both inpatients and outpatients. Hospitals prefer MCT solutions for their ability to provide early diagnosis, automated reporting, and seamless integration with hospital information systems. Large-scale deployment of MCT devices in hospitals ensures better patient management, reduced readmissions, and improved clinical outcomes. The availability of skilled cardiac professionals and advanced diagnostic protocols further supports hospital adoption.
The home healthcare segment is expected to witness the fastest growth from 2026 to 2033, fueled by increasing demand for remote cardiac monitoring, aging population, and patient preference for non-invasive care at home. Home healthcare providers and patients favor wearable MCT devices that offer real-time monitoring, AI-assisted alerts, and easy smartphone integration. Growing reimbursement support for home-based cardiac care and telehealth adoption is accelerating the penetration of MCT solutions in residential settings. The convenience, cost-effectiveness, and ability to reduce hospital visits make home healthcare the fastest-growing end-user segment in Europe.
Europe Mobile Cardiac Telemetry (MCT) Market Regional Analysis
- Germany dominated the Europe MCT market with the largest revenue share of 38.7% in 2025, characterized by advanced healthcare infrastructure, high adoption of digital health technologies, and a strong presence of key medical device players, with substantial growth in MCT adoption driven by innovations in wearable telemetry devices and AI-enabled arrhythmia detection
- Healthcare providers in Germany prioritize continuous, real-time cardiac monitoring for early detection of arrhythmias and improved patient outcomes, supporting the widespread deployment of MCT devices across hospitals, cardiac centers, and clinics
- This adoption is further reinforced by advanced healthcare infrastructure, high awareness of telehealth technologies, favorable reimbursement policies, and the presence of leading cardiac device manufacturers, establishing MCT as a preferred solution for both clinical and home-based cardiac care
The Germany Mobile Cardiac Telemetry (MCT) Market Insight
The Germany MCT market dominated Europe with the largest revenue share of 38.7% in 2025, fueled by high awareness of cardiovascular health and widespread adoption of digital healthcare solutions. Hospitals, cardiac centers, and home healthcare providers prioritize real-time arrhythmia detection and continuous monitoring to improve patient outcomes. The country’s advanced healthcare infrastructure, robust cardiac care facilities, and strong presence of medical device manufacturers support MCT adoption. Integration with AI-assisted analytics and telehealth platforms enhances diagnostic accuracy and patient management. Government initiatives promoting digital health and favorable reimbursement policies further drive market growth. The increasing focus on patient-centric, non-invasive wearable devices aligns with local healthcare expectations and contributes to market expansion.
France Mobile Cardiac Telemetry (MCT) Market Insight
The France MCT market is witnessing strong growth due to rising prevalence of cardiovascular diseases and increasing adoption of remote patient monitoring solutions. Hospitals and home healthcare providers are implementing MCT devices for early arrhythmia detection and better patient management. Government support for telemedicine programs and favorable reimbursement policies accelerate adoption across clinical and home care settings. Integration with digital health platforms and cloud-based monitoring enhances efficiency and real-time reporting. Patient awareness of the benefits of continuous cardiac monitoring drives demand in residential and outpatient care. The growing preference for wearable, user-friendly devices supports market expansion during the forecast period.
U.K. Mobile Cardiac Telemetry (MCT) Market Insight
The U.K. MCT market is expected to grow at a notable CAGR during the forecast period, driven by increasing cardiovascular disease prevalence and the adoption of telehealth solutions. Hospitals, cardiac centers, and home healthcare services prioritize continuous cardiac monitoring for early detection of arrhythmias and improved patient outcomes. Digital health initiatives and integration with wearable AI-enabled MCT devices enhance diagnostic accuracy and patient compliance. The U.K.’s strong healthcare infrastructure and high patient awareness support market growth. Favorable reimbursement policies and increasing telemonitoring adoption further stimulate expansion. The demand for convenient, non-invasive monitoring solutions in outpatient and home care settings is rising steadily.
Italy Mobile Cardiac Telemetry (MCT) Market Insight
The Italy MCT market is poised for robust growth due to rising cases of cardiovascular diseases and growing adoption of home-based cardiac monitoring. Hospitals and cardiac centers are leveraging MCT devices to reduce hospital visits and improve patient care. Government initiatives supporting digital health and telemedicine drive deployment in clinical and residential settings. Integration with cloud-based analytics and AI-enabled predictive tools enhances arrhythmia detection and patient management. Patient preference for wearable, non-invasive devices boosts adoption across home healthcare services. Increased awareness of the benefits of continuous monitoring contributes to market expansion during the forecast period.
Europe Mobile Cardiac Telemetry (MCT) Market Share
The Europe Mobile Cardiac Telemetry (MCT) industry is primarily led by well-established companies, including:
- iRhythm Technologies, Inc. (U.S.)
- BIOTRONIK SE & Co. KG (Germany)
- ZOLL Medical Corporation (U.S.)
- BioTelemetry, Inc., (U.S.)
- Medtronic (Ireland)
- Abbott (U.S.)
- GE Healthcare (U.S.)
- ScottCare Corporation (U.S.)
- Applied Cardiac Systems, Inc. (U.S.)
- Medicomp, Inc. (U.S.)
- Biotricity, Inc. (U.S.)
- Telerhythmics, LLC (U.S.)
- Boston Scientific Corporation (U.S.)
- NIHON KOHDEN CORPORATION (Japan)
- Hill-Rom Holdings, Inc. (U.S.)
- Spacelabs Healthcare (U.S.)
- Schiller AG (Switzerland)
- OSI Systems (U.S.)
- CardioNet, Inc. (U.S.)
- Preventice Solutions, Inc. (U.S.)
What are the Recent Developments in Europe Mobile Cardiac Telemetry (MCT) Market?
- In September 2025, Royal Philips unveiled a new enterprise‑wide telemetry platform for continuous cardiac monitoring (with the wearable Telemetry Monitor 5500), designed to improve alarm management, reduce hospital staff workload, and support scalable post-discharge remote monitoring through its MCOT offering. This represents a push to modernize in‑hospital telemetry systems and expand mobile cardiac telemetry capabilities in Europe and globally
- In November 2024, SmartCardia received FDA clearance for its 7‑lead live ECG patch and cloud platform for outpatient cardiac telemetry (OCT/MCT). While the clearance is from the U.S. regulator, the technology and cloud‑platform approach hold strong relevance for European cardiac telemetry trends, potentially influencing future regulatory submissions and market offerings in Europe
- In August 2024, iRhythm Technologies announced the commercial launch of its Zio® monitor and long‑term continuous (LTCM) ECG monitoring service across several European countries Austria, the Netherlands, Switzerland, and Spain. This patch‑based MCT system offers up to 14 days of continuous ECG monitoring, representing a major expansion of advanced ambulatory cardiac telemetry in Europe
- In May 2024, Royal Philips rolled out its wearable ePatch and AI‑driven analytics platform (Cardiologs) for ambulatory cardiac monitoring across 14 hospitals in Spain. The service is enabling extended wear Holter monitoring with up to 14‑day recording, improving patient comfort and enabling more effective detection of arrhythmias than traditional Holter monitors
- In December 2023, the next-generation version of the Zio monitor (by iRhythm) achieved CE Mark under the EU’s Medical Device Regulation (EU MDR), enabling its regulatory compliance and eventual commercial distribution across European markets. This paved the way for the 2024 European launch
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.