Europe Multiplex Assays Market
Market Size in USD Million
CAGR :
% 
 USD
609.30 Million
USD
1,202.53 Million
2024
2032
USD
609.30 Million
USD
1,202.53 Million
2024
2032
| 2025 –2032 | |
| USD 609.30 Million | |
| USD 1,202.53 Million | |
|
|
|
|
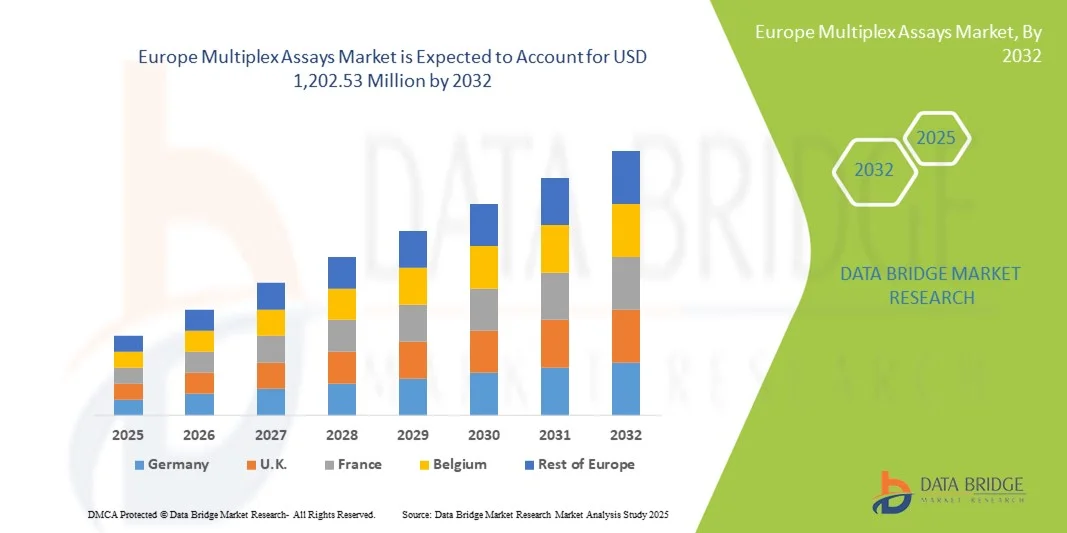
Europe Multiplex Assays Market Size
- The Europe Multiplex Assays market size was valued at USD 609.3 Million in 2024 and is expected to reach USD 1,202.53 Million by 2032, at a CAGR of 8.87% during the forecast period
- The market growth is largely fueled by the growing adoption and technological advancements in life sciences and diagnostics, leading to increased digitalization and automation across research and clinical laboratories
- Furthermore, rising demand for accurate, high-throughput, and cost-effective analytical solutions for biomarker detection, disease diagnosis, and drug development is positioning multiplex assays as the preferred analytical tool. These converging factors are accelerating the uptake of multiplex assays solutions, thereby significantly boosting the industry’s growth
Europe Multiplex Assays Market Analysis
- Multiplex assays, enabling simultaneous detection and quantification of multiple biomarkers or analytes in a single experiment, are increasingly critical in diagnostics, drug development, and research applications due to their efficiency, reduced sample volume requirements, and high-throughput capabilities
- The escalating demand for multiplex assays is primarily driven by the growing prevalence of chronic diseases, increasing adoption of personalized medicine, and the rising need for efficient and cost-effective diagnostic solutions
- Germany dominated the multiplex assays market with the largest revenue share of 41.3% in 2024, supported by advanced healthcare infrastructure, strong research and development investment, and the presence of key industry players. The country has seen substantial growth in Multiplex Assays adoption across clinical diagnostics, pharmaceutical research, and academic laboratories. Innovations from established biotech companies and emerging startups focusing on high-throughput, automated, and AI-integrated multiplex platforms have further strengthened market penetration
- The U.K. is expected to be the fastest-growing region in the multiplex assays market during the forecast period, with a CAGR from 2025 to 2032, driven by increasing investments in life sciences, expanding laboratory infrastructure, and rising adoption of advanced diagnostic technologies. Growth is further fueled by government initiatives supporting biotechnology and personalized medicine, along with the rising number of clinical trials and research programs utilizing multiplex platforms
- The PCR segment dominated the largest market revenue share of 45.3% in 2024, driven by widespread use in nucleic acid detection, clinical diagnostics, and research in the U.S. PCR-based assays offer high sensitivity, specificity, and rapid results, making them essential for laboratories and hospitals
Report Scope and Multiplex Assays Market Segmentation
|
Attributes |
Multiplex Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Multiplex Assays Market Trends
Enhanced Convenience Through Automation and Integrated Analytical Platforms
- A significant and accelerating trend in the multiplex assays market is the deepening integration with advanced data analytics platforms, automation technologies, and laboratory information management systems (LIMS). This fusion of technologies is significantly enhancing user convenience, data accuracy, and control over diagnostic workflows
- For instance, the Bio-Rad Bio-Plex 3D Suspension Array System seamlessly integrates with major analytical and LIMS software, allowing researchers to conduct, monitor, and interpret multiple assays simultaneously from a single interface. Similarly, Luminex xMAP platforms are designed for smooth integration with automated liquid handling systems, offering a high-throughput and streamlined multiplexing solution
- Automation integration in multiplex assays enables functions such as learning experimental patterns, optimizing workflow parameters, and providing more intelligent alerts based on assay performance metrics. For example, certain advanced multiplex systems utilize machine learning algorithms to improve assay calibration over time and generate intelligent notifications if abnormal sample results or variations are detected. Furthermore, integrated control capabilities offer researchers the ease of automated setup and execution, allowing them to perform complex multi-analyte testing with minimal manual intervention
- The seamless integration of multiplex assay instruments with digital laboratory systems and broader data management platforms facilitates centralized control over multiple stages of the analytical process. Through a unified interface, users can manage assay scheduling, sample tracking, data collection, and analysis simultaneously, creating an interconnected and automated laboratory environment
- This trend towards more intelligent, intuitive, and interconnected assay systems is fundamentally reshaping user expectations in life science research and clinical diagnostics. Consequently, companies such as Thermo Fisher Scientific and Quanterix Corporation are developing next-generation multiplex assay systems equipped with real-time data analysis capabilities, automatic assay optimization, and compatibility with centralized laboratory networks
- The demand for multiplex assays offering seamless integration, automation, and advanced analytical capabilities is growing rapidly across both research and clinical laboratories, as end-users increasingly prioritize efficiency, accuracy, and comprehensive data management within their testing operations
Europe Multiplex Assays Market Dynamics
Driver
Growing Need Due to Rising Disease Burden and Expanding Research Applications
- The increasing prevalence of chronic and infectious diseases, coupled with the growing demand for advanced diagnostic technologies, is a significant driver for the heightened adoption of multiplex assays
- For instance, in April 2024, Bio-Rad Laboratories, Inc. announced the launch of its next-generation Bio-Plex Pro Human Cytokine Screening Panel, expanding its coverage for inflammation and immune response research. Such developments by key industry players are expected to propel the growth of the multiplex assays market during the forecast period
- As healthcare providers and researchers seek enhanced testing efficiency and improved analytical precision, multiplex assays offer the ability to simultaneously detect and quantify multiple biomarkers within a single sample—providing a clear advantage over conventional single-analyte assays
- Furthermore, the growing emphasis on personalized medicine, precision oncology, and biomarker-based diagnostics is making multiplex assays an integral component of modern laboratory workflows. These assays enable comprehensive insights into complex biological systems and disease mechanisms, driving their adoption across pharmaceutical R&D, clinical diagnostics, and translational research
- The convenience of higher throughput, reduced sample consumption, faster turnaround times, and compatibility with existing laboratory infrastructure are key factors propelling the adoption of multiplex assay platforms. The rising trend toward automated laboratory systems and the increasing availability of user-friendly, ready-to-use multiplex kits further contribute to market growth
Restraint/Challenge
Concerns Regarding Data Complexity and High Implementation Costs
- Concerns surrounding the high complexity of data interpretation and the initial cost of multiplex assay systems pose significant challenges to broader market penetration. As these platforms rely on advanced instrumentation and bioinformatics tools, laboratories may face difficulties in managing large-scale, multi-dimensional datasets and ensuring data reproducibility
- For instance, reports of variability in assay performance due to sample quality, reagent stability, or platform calibration have made some laboratories cautious about adopting multiplex systems for critical diagnostic applications
- Addressing these challenges through improved assay standardization, robust data analytics software, and comprehensive technical support is crucial for building user confidence
- Companies such as Thermo Fisher Scientific and MilliporeSigma emphasize their advanced validation processes, standardized protocols, and quality assurance measures to reassure end users. In addition, the relatively high initial investment for high-throughput multiplex platforms compared to conventional ELISA or PCR systems can be a barrier for smaller laboratories or academic institutions with limited budgets. While compact and modular assay kits from companies such as Abcam and Quansys Biosciences have become more affordable, premium systems with automated sample processing or integrated digital analysis modules often carry higher costs.
- Although the cost gap is gradually narrowing, the perception of multiplex assays as high-end technology can still hinder widespread adoption, especially among cost-sensitive end users
- Overcoming these limitations through enhanced data interpretation tools, training programs, and the development of cost-effective multiplex solutions will be vital for sustaining long-term market expansion
Europe Multiplex Assays Market Scope
The market is segmented on the basis of products and services, type, technology, application, and end user.
- By Products and Services
On the basis of Products and Services, the Multiplex Assays market is segmented into Reagents and Consumables, Instruments and Accessories, and Software and Services. The Reagents and Consumables segment dominated the largest market revenue share of 44.1% in 2024, driven by its critical role in assay performance, reproducibility, and reliability. U.S. laboratories and hospitals prioritize high-quality reagents due to their compatibility with multiple multiplex platforms and essential role in clinical diagnostics and R&D. The segment benefits from a wide variety of assay kits, optimized for sensitivity, throughput, and accuracy. Strong presence of leading suppliers in Europe strengthens adoption. Continuous innovation in reagents ensures improved assay performance and workflow efficiency. High-throughput applications and large-scale studies sustain consistent demand. Regulatory compliance, standardization, and technical support reinforce market leadership. Adoption in personalized medicine, infectious disease testing, and biomarker discovery further drives growth. Reagents are considered essential for both academic research and commercial laboratories. Partnerships between manufacturers and research institutes enhance availability. Cost-effectiveness, ease of integration, and reliability maintain segment dominance.
The Instruments and Accessories segment is expected to witness the fastest CAGR of 22.5% from 2025 to 2032, with Canada emerging as the fastest-growing market. Growing adoption of automated multiplex instruments, microplate readers, and accessories in research labs and biotech startups drives this growth. Instruments enable high-throughput analysis, improved sensitivity, and integration with laboratory software. Canadian laboratories benefit from government funding and modernization of research infrastructure. Increasing demand in clinical trials, pharmaceutical R&D, and academic research accelerates adoption. The segment also gains traction due to modular instrument design and compatibility with multiple assay types. Integration with data analytics and workflow automation enhances efficiency. Emerging biotech companies in Canada are rapidly adopting these platforms for multi-analyte detection. Service agreements, maintenance, and technical support further strengthen market growth. Instruments improve reproducibility, reduce sample volume requirements, and optimize laboratory workflow. Adoption in translational research, immunology, and oncology applications drives segment expansion.
- By Type
On the basis of Type, the Multiplex Assays market is segmented into Nucleic Acid Multiplex Assays, Protein Multiplex Assays, Cell-Based Multiplex Assays, and Others. The Nucleic Acid Multiplex Assays segment dominated the largest market revenue share of 42.7% in 2024, driven by high demand in genetic testing, infectious disease detection, and clinical diagnostics in the U.S. Laboratories prefer nucleic acid assays due to their sensitivity, specificity, and ability to analyze multiple targets simultaneously. Integration with automated PCR and real-time platforms ensures rapid turnaround and high reproducibility. Large-scale epidemiological studies and clinical trials further boost demand. Continuous innovation in assay chemistry and workflow optimization reinforces adoption. U.S. hospitals and research institutes prioritize nucleic acid assays for routine testing and advanced research. High-throughput and multi-target capabilities improve efficiency and cost-effectiveness. Strategic partnerships between assay providers and labs enhance product availability. Standardization, regulatory compliance, and technical support strengthen segment dominance. Ongoing R&D activities and adoption in personalized medicine maintain leadership.
The Protein Multiplex Assays segment is expected to witness the fastest CAGR of 21.9% from 2025 to 2032, with Canada emerging as the fastest-growing market. Protein assays are increasingly used in biomarker discovery, drug development, and clinical proteomics. Advanced platforms, including microarrays and flow cytometry, enhance throughput and sensitivity. Canadian laboratories benefit from government support, expanding research infrastructure, and rising awareness of multiplex proteomics. Integration with automation and data analytics streamlines workflows. Startups and commercial labs adopt protein assays for scalable, multi-analyte testing. Standardized protocols, reproducibility, and user-friendly platforms accelerate adoption. High demand in immunology, oncology, and translational research strengthens growth. Collaborative research projects between academic and commercial labs support expansion. Cost-effectiveness and efficiency drive adoption. Emerging applications in diagnostics and drug screening increase uptake. Technical support and instrument compatibility facilitate segment growth.
- By Technology
On the basis of Technology, the Multiplex Assays market is segmented into Protein Microarray, Polymerase Chain Reaction (PCR), Multiplex Real-Time PCR, Flow Cytometry, Fluorescence Detection, Luminescence, and Others. The PCR segment dominated the largest market revenue share of 45.3% in 2024, driven by widespread use in nucleic acid detection, clinical diagnostics, and research in the U.S. PCR-based assays offer high sensitivity, specificity, and rapid results, making them essential for laboratories and hospitals. Continuous innovations in real-time and multiplex PCR platforms enhance throughput. Integration with laboratory information management systems and automated workflows strengthens adoption. Large-scale genomic and infectious disease studies further increase demand. Regulatory approvals, standardization, and technical support contribute to leadership. PCR assays enable early detection, cost-effective testing, and accurate quantification. U.S. research institutes and commercial laboratories prioritize PCR for high-throughput applications. Partnerships with instrument manufacturers reinforce adoption. Ongoing R&D and training programs maintain segment dominance.
The Flow Cytometry segment is expected to witness the fastest CAGR of 23.1% from 2025 to 2032, with Canada leading growth. Flow cytometry enables high-throughput cell-based analysis, immunophenotyping, and drug discovery applications. Canadian adoption is rising due to expanding biotech and research infrastructure, government funding, and increased focus on advanced cell-based studies. Integration with multiplex assay platforms and bioinformatics tools enhances efficiency and accuracy. Standardized protocols, technical support, and modular platforms encourage adoption. High sensitivity, specificity, and multi-marker analysis drive segment growth. Emerging applications in immunotherapy and oncology increase demand. Workflow optimization and reproducibility make flow cytometry ideal for research labs. Startups and commercial labs adopt cytometry platforms for scalable multi-analyte testing. Cost-effectiveness, ease of use, and automation support expansion. Collaborative research and increasing R&D investments further strengthen market penetration.
- By Application
On the basis of Application, the Multiplex Assays market is segmented into Clinical Diagnostics and Research & Development. The Clinical Diagnostics segment accounted for the largest market revenue share of 43.8% in 2024, driven by the adoption of multiplex assays in hospitals, clinical laboratories, and research institutions in the U.S. High demand for rapid, accurate multi-analyte testing supports adoption. Integration with automated workflows, data management systems, and LIMS enhances operational efficiency. Large-scale diagnostic applications, regulatory compliance, and robust technical support reinforce dominance. U.S. clinical laboratories prioritize high-throughput assays for infectious disease detection, oncology, and personalized medicine. Multi-target detection capabilities reduce cost and turnaround time. Continuous innovation in assay platforms ensures reproducibility and reliability. Strategic partnerships between assay developers and healthcare providers strengthen deployment. The segment benefits from government support, clinical trials, and advanced hospital infrastructure. Expansion of diagnostic networks increases accessibility. Overall, clinical diagnostics remain the dominant application in Europe.
The Research & Development segment is expected to witness the fastest CAGR of 22.7% from 2025 to 2032, with Canada as the fastest-growing market. Multiplex assays are increasingly used in academic research, pharmaceutical R&D, and biotech startups for multi-analyte detection, biomarker discovery, and drug development. High-throughput capabilities, workflow automation, and data integration enhance research efficiency. Canadian investment in laboratory infrastructure, government funding, and training programs support rapid adoption. Standardization, reproducibility, and integration with digital tools drive segment growth. Emerging applications in proteomics, genomics, and translational research increase demand. Flexibility, cost-effectiveness, and scalability make multiplex assays attractive for research labs. Collaborative projects with academic and commercial institutions accelerate adoption. Technical support and instrument compatibility ensure reliable operation. Startups and small labs contribute to rapid expansion. Workflow optimization and user-friendly platforms further encourage uptake. Overall, Research & Development is the fastest-growing application in Europe.
- By End User
On the basis of End User, the Multiplex Assays market is segmented into Hospitals, Clinical Laboratories, Pharmaceutical and Biotechnology Companies, Research Institutes, and Others. The Hospitals segment dominated the largest market revenue share of 43.5% in 2024, driven by the critical role of multiplex assays in patient diagnostics, disease monitoring, and clinical research. U.S. hospitals and medical centers prioritize high-throughput, multi-analyte platforms due to their ability to deliver rapid, accurate results across multiple tests. The segment benefits from integration with laboratory information management systems, automated workflows, and AI-assisted data analysis. Strong infrastructure, regulatory compliance, and established technical support reinforce market leadership. Continuous investment in hospital laboratories ensures sustained adoption. Multiplex assays enable early detection, personalized treatment, and efficient resource utilization. Partnerships between assay providers and hospital networks enhance accessibility and reliability. Ongoing clinical trials, translational research, and incorporation of novel biomarkers support growth. Hospitals also leverage multiplex platforms for infectious disease screening, oncology diagnostics, and immunology testing. The segment remains essential for both routine diagnostics and specialized clinical applications. Strong presence of leading assay suppliers in the U.S. further drives adoption.
The Research Institutes segment is expected to witness the fastest CAGR of 22.3% from 2025 to 2032, with the United Kingdom emerging as the fastest-growing market. Academic and research institutions increasingly adopt multiplex assay platforms for biomarker discovery, drug development, and multi-analyte research. The segment benefits from government funding, expanding laboratory infrastructure, and initiatives supporting life sciences innovation in the UK. High-throughput, automated platforms streamline workflows, reduce sample requirements, and improve reproducibility. Integration with digital analytics, AI-assisted interpretation, and multi-site collaboration accelerates adoption. Research institutes prioritize flexible, modular instruments to support diverse experimental needs. Emerging biotech startups and translational research projects drive segment expansion. Standardized protocols, training programs, and technical support further enhance usability. Growing focus on precision medicine, immunology, and genomics fuels demand. Research institutes leverage multiplex assays to improve data accuracy, scalability, and efficiency. Collaborative programs between academia and industry strengthen market penetration. Cost-effectiveness, ease of integration, and multi-analyte detection capabilities position research institutes as the fastest-growing end user segment in Europe.
Europe Multiplex Assays Market Regional Analysis
- The Germany multiplex assays market dominated Europe with the largest revenue share of 41.3% in 2024, supported by advanced healthcare infrastructure, strong R&D investment, and the presence of key industry players. Germany has experienced substantial growth in multiplex assay adoption across clinical diagnostics, pharmaceutical research, and academic laboratories
- Leading biotech companies and emerging startups focusing on high-throughput, automated, and AI-integrated multiplex platforms have strengthened market penetration. The country’s well-developed infrastructure, coupled with strong emphasis on innovation and technological advancement, promotes the adoption of multiplex assays in hospitals, research institutions, and commercial laboratories
- Laboratories prioritize multi-analyte detection to improve workflow efficiency, reproducibility, and diagnostic accuracy. Integration with digital solutions and laboratory information management systems supports streamlined operations
U.K. Multiplex Assays Market Insight
The U.K. multiplex assays market is anticipated to grow at a noteworthy CAGR of 22.1% from 2025 to 2032, driven by increasing investments in life sciences, expanding laboratory infrastructure, and growing adoption of advanced diagnostic technologies. Rising government support for biotechnology and personalized medicine, coupled with the increasing number of clinical trials and research programs, further accelerates growth. U.K. laboratories and research institutions are adopting high-throughput, automated multiplex platforms for clinical diagnostics, pharmaceutical R&D, and translational research. The focus on reducing assay turnaround time, improving reproducibility, and enabling multi-analyte detection supports segment expansion. Collaborations between biotech startups, academic institutions, and hospitals enhance adoption. Integration with digital systems and AI-assisted data analysis improves operational efficiency and workflow management. Increasing awareness of precision medicine and biomarker discovery drives further market demand.
Germany Multiplex Assays Market Insight
The Germany multiplex assays market dominated Europe with the largest revenue share of 41.3% in 2024, supported by advanced healthcare infrastructure, strong R&D investment, and the presence of key industry players. Germany has experienced substantial growth in multiplex assay adoption across clinical diagnostics, pharmaceutical research, and academic laboratories. Leading biotech companies and emerging startups focusing on high-throughput, automated, and AI-integrated multiplex platforms have strengthened market penetration. The country’s well-developed infrastructure, coupled with strong emphasis on innovation and technological advancement, promotes the adoption of multiplex assays in hospitals, research institutions, and commercial laboratories. Laboratories prioritize multi-analyte detection to improve workflow efficiency, reproducibility, and diagnostic accuracy. Integration with digital solutions and laboratory information management systems supports streamlined operations.
Europe Multiplex Assays Market Share
The Multiplex Assays industry is primarily led by well-established companies, including:
The Multiplex Assays industry is primarily led by well-established companies, including:
• Thermo Fisher Scientific Inc(U.S.)
• Bio-Rad Laboratories (U.S.)
• BD (U.S.)
• PerkinElmer (U.S.)
• Agilent Technologies (U.S.)
• Illumina, Inc. (U.S.)
• Quanterix Corporation (U.S.)
• Meso Scale Diagnostics (U.S.)
• Myriad RBM (U.S.)
• Randox Laboratories (U.K.)
• Olink Proteomics (Sweden)
• Abcam (U.K.)
• Cell Signaling Technology (U.S.)
Latest Developments in Europe Multiplex Assays Market
- In November 2021, QuantuMDx secured a USD 20.0 million equity investment from Vita Spring IVD Fund, L.P., a Hong Kong-based venture capital firm focused on early and growth-stage medical companies. This funding aims to further develop the Q-POC platform, enhancing its multiplexing capabilities and expanding its assay menu. The company also entered into a cooperation agreement with Sansure Biotech to accelerate the commercialization of the platform in China and other Southeast Asian markets
- In June 2022, Illumina announced the addition of a companion diagnostic (CDx) indication to its CE-marked in vitro diagnostic TruSight Oncology Comprehensive (EU) test. This addition enables the test to be used for identifying patients with ROS1 fusion-positive non-small cell lung cancer (NSCLC) or patients with NTRK fusion-positive cancers, for whom treatment with Roche’s Rozlytrek (entrectinib) may be appropriate
- In April 2023, Bio-Rad Laboratories announced a collaboration with Seegene to develop and commercialize infectious disease molecular diagnostic products for the U.S. market. Under the agreement, Seegene will provide diagnostic tests for use on Bio-Rad's CFX96™ Dx Real-Time PCR System, pending clinical development and approval from the U.S. Food & Drug Administration (FDA)
- In March 2024, Co-Diagnostics, Inc. announced that its joint venture in India, CoSara Diagnostics Pvt. Ltd., received regulatory clearance from the Central Drugs Standard Control Organization (CDSCO) to manufacture and sell its SARAPLEX Influenza Multiplex (IFM) Test Kit. This real-time PCR multiplex test is designed using Co-Dx Co-Primers and is approved for use in diagnostic procedures in clinical laboratories
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.