Europe Parenteral Packaging Market
Market Size in USD Billion
CAGR :
% 
 USD
8.02 Billion
USD
13.07 Billion
2025
2033
USD
8.02 Billion
USD
13.07 Billion
2025
2033
| 2026 –2033 | |
| USD 8.02 Billion | |
| USD 13.07 Billion | |
|
|
|
|
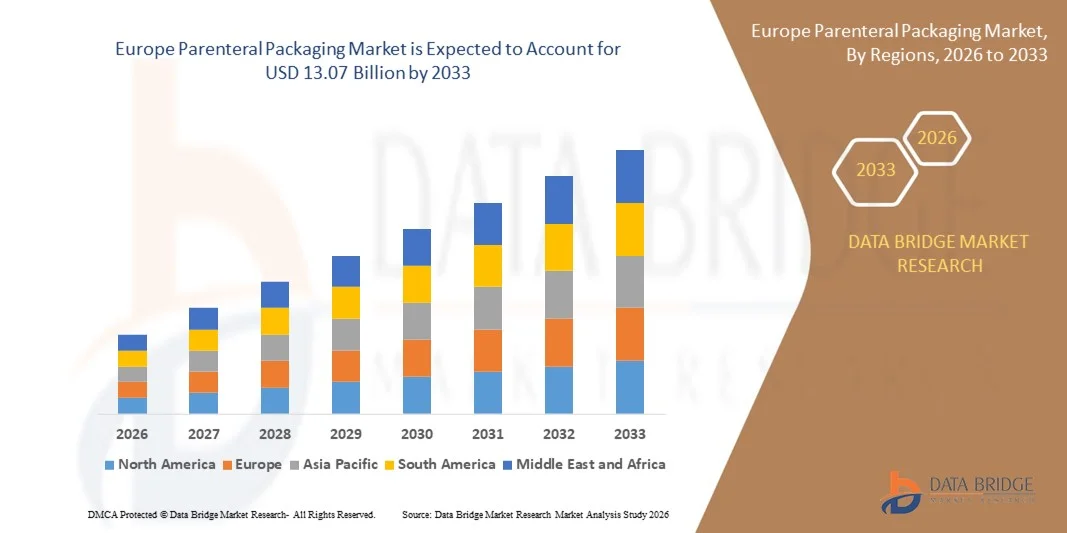
Europe Parenteral Packaging Market Size
- The Europe parenteral packaging market size was valued at USD 8.02 billion in 2025 and is expected to reach USD 13.07 billion by 2033, at a CAGR of 6.3% during the forecast period
- The market growth is largely fueled by the rising demand for safe, efficient, and advanced drug delivery systems, along with technological advancements in packaging materials such as glass, polymer, and cyclic olefin polymers, enabling enhanced drug stability and patient safety
- Furthermore, increasing production and administration of vaccines, biologics, and high-potency injectable drugs are driving the need for reliable, sterile, and scalable parenteral packaging solutions. These converging factors are accelerating the adoption of innovative packaging formats, thereby significantly boosting the industry's growth
Europe Parenteral Packaging Market Analysis
- Parenteral packaging, including vials, pre-filled syringes, ampoules, and ready-to-use systems, is becoming a critical component of modern pharmaceutical manufacturing and healthcare delivery due to its ability to maintain drug sterility, ensure accurate dosing, and support biologic and specialty drug administration
- The escalating demand for parenteral packaging is primarily fueled by the growth of injectable therapies, increasing prevalence of chronic and infectious diseases, and a rising preference for pre-filled and ready-to-use formats that enhance patient safety, operational efficiency, and compliance with regulatory standards
- Germany dominated the parenteral packaging market in 2025, due to a strong pharmaceutical and biotechnology sector, high adoption of advanced parenteral systems, and stringent regulatory standards ensuring drug safety and quality
- U.K. is expected to be the fastest growing country in the parenteral packaging market during the forecast period due to growing adoption of advanced injectable therapies, rising biologics production, and expanding healthcare infrastructure.
- Vials segment dominated the market with a market share of 43.3% in 2025, due to their widespread use in injectable formulations and strong compatibility with automated filling and sealing systems. Pharmaceutical manufacturers prefer vials for their ability to maintain sterility and long shelf life, ensuring safety and efficacy of injectable drugs. The dominance is further supported by the extensive adoption of vials in vaccines, biologics, and therapeutic injectables, enhancing their demand across hospitals and clinics. Vials also provide flexibility for single-dose and multi-dose formulations, meeting diverse clinical and commercial requirements
Report Scope and Parenteral Packaging Market Segmentation
|
Attributes |
Parenteral Packaging Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Parenteral Packaging Market Trends
Growing Adoption of Pre-Filled and Ready-to-Use Parenteral Systems
- A significant trend in the parenteral packaging market is the increasing adoption of pre-filled and ready-to-use systems, driven by the growing need for patient-centric drug delivery and operational efficiency in healthcare settings. These formats enhance dosing accuracy, reduce medication errors, and simplify administration in hospitals, clinics, and homecare environments
- For instance, BD (Becton, Dickinson and Company) and Terumo Pharmaceutical Solutions supply high-quality pre-filled syringes and ready-to-use polymer syringes that are widely used for vaccines, biologics, and specialty injectables. Such solutions improve drug safety, streamline clinical workflows, and strengthen manufacturer reliability across markets
- The adoption of pre-filled and ready-to-use systems is rising rapidly in immunization programs and chronic disease management, where minimizing preparation time and contamination risks is critical. This is positioning parenteral packaging as a key enabler for safer, more efficient healthcare delivery
- Pharmaceutical manufacturers are increasingly integrating advanced filling and sealing technologies to support large-scale production of pre-filled and ready-to-use systems, further driving market growth
- Hospitals and ambulatory care services are showing preference for these formats due to ease of handling, reduced storage requirements, and compatibility with automated dispensing systems
- The growing focus on biologics and high-potency injectable therapies is reinforcing demand for pre-filled and ready-to-use packaging, as these formats ensure product integrity, maintain sterility, and meet stringent regulatory standards
Europe Parenteral Packaging Market Dynamics
Driver
Rising Demand for Biologics and Injectable Therapies
- The increasing production and consumption of biologics, vaccines, and high-potency injectable drugs is a major driver for the parenteral packaging market, as these therapies require secure, sterile, and reliable packaging solutions that preserve drug stability and efficacy
- For instance, Gerresheimer and SCHOTT Pharma provide specialized vials and polymer-based pre-filled systems designed for sensitive formulations, including mRNA vaccines and monoclonal antibodies. These solutions enhance product safety, facilitate large-scale distribution, and support efficient administration in clinical and homecare settings
- The rising prevalence of chronic diseases, infectious diseases, and specialty therapies is encouraging healthcare providers to adopt injectable treatments, thereby increasing the demand for high-quality parenteral packaging
- Pharmaceutical companies are investing in innovative packaging solutions to meet stringent global regulatory requirements while ensuring patient safety, operational efficiency, and minimal drug wastage
- Integration of automated filling, sealing, and inspection technologies allows manufacturers to scale production efficiently, support growing biologic pipelines, and meet the demand for ready-to-use and pre-filled formats
Restraint/Challenge
High Manufacturing and Regulatory Compliance Costs
- The parenteral packaging market faces significant challenges due to the high costs associated with producing sterile, high-quality packaging systems that comply with rigorous regulatory standards across multiple regions
- For instance, companies such as Terumo Pharmaceutical Solutions and BD invest heavily in advanced sterilization, inspection, and quality control processes to meet global pharmacopeia requirements, which increases operational expenses and complexity
- Ensuring drug stability, sterility, and compatibility with sensitive biologics requires advanced materials, precision engineering, and specialized equipment, further raising production costs and limiting cost flexibility
- The constantly evolving regulatory landscape across different countries adds to the compliance burden, requiring continuous monitoring, testing, and documentation to maintain market approval
- Scaling production while maintaining consistent quality, preventing contamination, and meeting regulatory standards remains a critical constraint, impacting profitability and limiting the ability of manufacturers to lower prices or rapidly expand capacity
Europe Parenteral Packaging Market Scope
The market is segmented on the basis of type, packaging type, order type, dosage type, distribution channel, therapeutic areas, and end user.
- By Type
On the basis of type, the parenteral packaging market is segmented into ampoules, pre-filled syringes, vials, bottles, cartridges, bags, ready-to-use systems, and others. The vials segment dominated the market with the largest revenue share in 2025, driven by their widespread use in injectable formulations and strong compatibility with automated filling and sealing systems. Pharmaceutical manufacturers prefer vials for their ability to maintain sterility and long shelf life, ensuring safety and efficacy of injectable drugs. The dominance is further supported by the extensive adoption of vials in vaccines, biologics, and therapeutic injectables, enhancing their demand across hospitals and clinics. Vials also provide flexibility for single-dose and multi-dose formulations, meeting diverse clinical and commercial requirements.
The pre-filled syringes segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by the rising demand for patient-centric drug delivery and ease of self-administration. For instance, companies such as Becton Dickinson are expanding their pre-filled syringe offerings to support biologics and monoclonal antibody therapies. Pre-filled syringes reduce dosing errors, enhance convenience, and improve patient compliance, making them increasingly preferred in home healthcare settings. Their adoption is also growing in emerging economies due to cost-effective manufacturing and reduced medication wastage. The combination of safety, convenience, and regulatory acceptance drives the rapid expansion of this segment.
- By Packaging Type
On the basis of packaging type, the market is segmented into smaller volume parenteral packaging and large volume parenteral packaging. Smaller volume parenteral packaging held the largest market share in 2025, driven by its extensive application in vaccines, insulin, and biologics requiring precise dosages. Smaller containers ensure reduced contamination risk and allow controlled administration in clinical and outpatient settings. Hospitals and ambulatory care centers prefer smaller volume formats for their ease of handling, storage, and rapid administration. The segment also benefits from technological advancements in glass and polymer-based materials that enhance drug stability and packaging integrity.
Large volume parenteral packaging is expected to witness the fastest growth, supported by rising demand in intravenous fluids, parenteral nutrition, and large-dose biologics. For instance, Fresenius Kabi has focused on expanding its line of large-volume bags for infusion therapies, catering to hospitals and homecare markets. Large-volume packaging improves efficiency in high-volume drug administration and reduces the frequency of dosing interventions. Growth in chronic disease management and critical care settings further propels demand. The segment’s scalability and suitability for hospital use drive its rapid expansion.
- By Order Type
On the basis of order type, the parenteral packaging market is categorized into customized orders and standard orders. Standard orders dominated the market in 2025, driven by high-volume pharmaceutical production and routine injectable formulations. Standardized packaging ensures faster production, lower costs, and easier regulatory compliance for pharmaceutical manufacturers. These orders cater to consistent market demand and facilitate global supply chain management. The segment benefits from widespread adoption across vaccines, biologics, and small-molecule drugs, enhancing efficiency in production and distribution.
Customized orders are projected to witness the fastest growth due to rising demand for personalized medicine and specialty biologics requiring tailored packaging solutions. For instance, West Pharmaceutical Services offers bespoke packaging solutions to meet specific drug compatibility and regulatory requirements. Customized packaging improves patient safety, reduces drug wastage, and supports niche therapeutic areas. Pharmaceutical companies increasingly opt for customized formats to differentiate products and comply with specific end-user needs. The segment’s growth is accelerated by the expanding biologics and specialty drug pipeline.
- By Dosage Type
On the basis of dosage type, the market is segmented into single dosage and multiple dosage. Single dosage dominated the market in 2025, driven by its higher safety, lower contamination risk, and convenience for outpatient administration. Single-dose formats are widely adopted for vaccines, insulin, and high-potency biologics, ensuring accurate dosing and minimal wastage. Hospitals and clinics favor single-dose packaging to simplify handling and reduce errors in drug administration. Technological advancements in materials and sealing systems further strengthen this segment’s dominance.
Multiple dosage is expected to witness the fastest growth due to increasing demand for cost-effective solutions in high-volume treatments and hospital-based administration. For instance, BD’s multi-dose vial systems support chronic disease management and mass immunization programs. Multiple-dose formats reduce storage requirements and overall treatment costs while maintaining sterility and efficacy. The segment is increasingly used in critical care and large-scale therapeutic applications, driving market expansion.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into direct sales, medical stores/pharmacies, e-commerce, and others. Direct sales dominated the market in 2025, driven by strong relationships between pharmaceutical manufacturers and hospitals, clinics, and contract research organizations. Direct distribution ensures product integrity, compliance with cold chain requirements, and timely delivery of critical parenteral drugs. Manufacturers also leverage direct sales to maintain control over pricing, quality, and regulatory adherence.
E-commerce is projected to witness the fastest growth, supported by increasing adoption of digital platforms for pharmaceutical procurement. For instance, McKesson’s online distribution channels provide hospitals and pharmacies with easy access to parenteral packaging products. E-commerce offers convenience, faster order processing, and wider product visibility, especially in emerging markets. The expansion of online pharmaceutical retail and B2B procurement solutions accelerates this segment’s growth.
- By Therapeutic Areas
On the basis of therapeutic areas, the market is segmented into cardiovascular/metabolic, obstetrics/gynecology, dermatology, endocrinology and metabolic disease, gastroenterology, ophthalmology, pain management, rare diseases, infectious diseases, and others. Infectious diseases dominated the market in 2025, driven by high demand for vaccines and injectable antibiotics in both developed and emerging economies. Hospitals, clinics, and vaccination programs prefer parenteral formats to ensure rapid efficacy and controlled dosing. The segment benefits from government immunization initiatives and increasing focus on infectious disease management globally.
Rare diseases are expected to witness the fastest growth, fueled by rising development of orphan drugs and specialty biologics requiring precise parenteral delivery. For instance, Novartis has expanded its parenteral solutions for rare disease therapies, supporting hospital and homecare administration. The growing focus on targeted therapies and personalized medicine drives demand for small-volume, high-potency injectable formats. This segment’s growth is accelerated by regulatory incentives and increased investment in rare disease treatment pipelines.
- By End User
On the basis of end user, the market is segmented into pharmaceutical manufacturers, hospitals, dispensaries, clinics, ambulatory services, contract research organizations, and others. Hospitals dominated the market in 2025, driven by high patient volumes and extensive use of parenteral drugs for critical care, surgery, and outpatient treatment. Hospitals require diverse packaging formats to support single-dose, multi-dose, and large-volume therapies while maintaining sterility and regulatory compliance. The segment also benefits from rapid adoption of advanced packaging technologies and automated dispensing systems.
Pharmaceutical manufacturers are projected to witness the fastest growth due to increasing outsourcing of packaging requirements and expansion of biologics and vaccine production. For instance, Pfizer has scaled up its parenteral packaging operations to meet global vaccine demand efficiently. Manufacturers increasingly prefer pre-filled syringes, vials, and ready-to-use systems to optimize production and distribution. The focus on high-quality, scalable packaging solutions for new drug pipelines accelerates growth in this segment.
Europe Parenteral Packaging Market Regional Analysis
- Germany dominated the parenteral packaging market with the largest revenue share in 2025, driven by a strong pharmaceutical and biotechnology sector, high adoption of advanced parenteral systems, and stringent regulatory standards ensuring drug safety and quality. The country’s focus on innovation, combined with widespread implementation of automated filling and inspection technologies, continues to accelerate adoption of pre-filled syringes, vials, and ready-to-use packaging formats
- The presence of leading global and domestic packaging solution providers, robust partnerships with pharmaceutical manufacturers, and advanced production facilities reinforces Germany’s leadership in this sector. Collaborations between major packaging companies and German pharmaceutical distributors, along with government-supported initiatives for biologics and vaccine production, enhance accessibility and improve production efficiency
- High investment in R&D, rising demand for biologics and specialty injectables, and focus on patient-centric packaging solutions further support Germany’s dominant position. Adoption of advanced materials, precision engineering, and scalable manufacturing processes contributes to sustained growth. Germany’s combination of strong policy support, technological infrastructure, and established pharmaceutical ecosystem secures its leadership in Europe’s parenteral packaging market
U.K. Parenteral Packaging Market Insight
The U.K. market is projected to record the fastest CAGR in the Europe parenteral packaging market during 2026–2033, supported by growing adoption of advanced injectable therapies, rising biologics production, and expanding healthcare infrastructure. For instance, collaborations between companies such as Gerresheimer and local pharmaceutical manufacturers to supply pre-filled syringes and polymer vials enhance production efficiency and market penetration. Increasing focus on patient safety, operational efficiency, and regulatory compliance is accelerating the adoption of ready-to-use and pre-filled packaging systems. Investments in automated filling and sealing technologies, combined with partnerships between global packaging providers and U.K.-based pharma companies, are enabling scalable, high-quality production. Strong government regulations, growing biologics pipelines, and rising demand for sterile packaging solutions further reinforce the U.K.’s position as the fastest-growing market in the region.
France Parenteral Packaging Market Insight
France is expected to experience steady growth from 2026 to 2033, driven by a robust pharmaceutical sector, rising biologics and vaccine production, and increasing adoption of pre-filled and ready-to-use parenteral packaging formats. The country’s strong regulatory framework and ongoing investments in advanced manufacturing technologies are encouraging pharmaceutical companies to adopt high-quality, patient-centric packaging solutions. Partnerships between French pharmaceutical manufacturers and global packaging suppliers, along with awareness programs highlighting safety and operational efficiency, are improving product accessibility. Expanding hospital and clinic networks, integration of automated production processes, and increasing demand for specialty injectables support consistent market expansion. France’s emphasis on drug safety, quality compliance, and technological advancement ensures stable growth and strengthens its regional presence within Europe’s parenteral packaging market.
Europe Parenteral Packaging Market Share
The parenteral packaging industry is primarily led by well-established companies, including:
- SCHOTT AG (Germany)
- Smithers (U.S.)
- WILCO AG (Switzerland)
- Genesis Packaging Technologies (U.S.)
- Baxter (U.S.)
- RONDO BURGDORF AG (Switzerland)
- ISOVOLTA AG (Austria)
- NNE (Denmark)
- Tekni-Plex, Inc (U.S.)
- Catalent, Inc (U.S.)
- Wipak Group (Finland)
- ProAmpac (U.S.)
- Nolato AB (Sweden)
- SiO2 Materials Science (U.S.)
Latest Developments in Europe Parenteral Packaging Market
- In December 2023, SCHOTT Pharma announced the expansion of its operations through the establishment of a new production facility in Serbia. This strategic investment aims to significantly scale up the company’s manufacturing capacity to meet the rising global demand for parenteral packaging. The development is expected to enhance supply chain efficiency, reduce lead times, and strengthen SCHOTT’s competitive foothold in key international markets. The new facility also underscores the company’s commitment to supporting the growing pharmaceutical and biotech sectors with high-quality, reliable packaging solutions
- In 2023, Gerresheimer introduced Cyclic Olefin Polymer (COP) vials specifically designed for sensitive biologics, including mRNA-based drugs. These vials offer enhanced drug safety by providing superior chemical stability and reduced interaction with high-potency formulations, ensuring the integrity of next-generation therapeutics. The innovation supports the growing need for advanced materials in parenteral packaging and allows pharmaceutical companies to address challenges associated with biologic and vaccine delivery. This launch reinforces Gerresheimer’s position as a leading provider of technologically advanced packaging solutions in the global market
- In 2023, BD (Becton, Dickinson and Company) launched a prefilled flush syringe with an integrated disinfection unit, aimed at streamlining drug administration in clinical settings. The solution improves operational safety by minimizing contamination risks during injections and enhances efficiency by reducing the steps required for preparation. This innovation expands BD’s portfolio in the parenteral packaging sector and supports healthcare providers in delivering safer and more reliable therapies, particularly in high-volume hospital and outpatient environments
- In 2022, Terumo Pharmaceutical Solutions (TPS) introduced a ready-to-fill polymer syringe specifically tailored for high-volume biotech drugs. This development addresses critical industry challenges, such as maintaining drug stability, ensuring sterility, and accommodating complex biologic formulations. The solution provides a robust and scalable packaging option for pharmaceutical manufacturers, strengthening TPS’s position in the competitive biopharmaceutical packaging segment and enabling more efficient production of advanced injectable therapies
- In 2021, SCHOTT AG began the construction of a second melting tank for pharmaceutical glass tubing to expand production capacity. This expansion is designed to meet the rising demand for high-quality parenteral glass packaging, particularly for vaccines, biologics, and injectable drugs. The additional capacity enables SCHOTT to better serve global pharmaceutical clients while supporting its long-term growth strategy in the healthcare sector. The initiative reflects the company’s ongoing investment in infrastructure to ensure reliability, precision, and scalability in glass packaging solutions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Europe Parenteral Packaging Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Europe Parenteral Packaging Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Europe Parenteral Packaging Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.