Europe Pcr Multiplex Assays Market
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
2.81 Billion
2025
2033
USD
1.70 Billion
USD
2.81 Billion
2025
2033
| 2026 –2033 | |
| USD 1.70 Billion | |
| USD 2.81 Billion | |
|
|
|
|
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Size
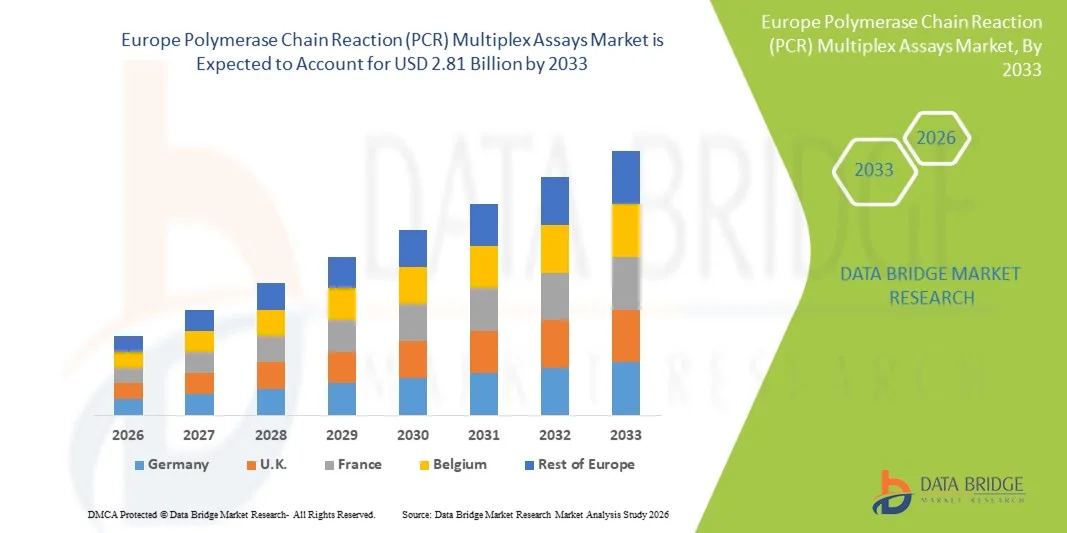
- The Europe polymerase chain reaction (PCR) multiplex assays market size was valued at USD 1.70 billion in 2025 and is expected to reach USD 2.81 billion by 2033, at a CAGR of 6.50% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced molecular diagnostic techniques, rising prevalence of infectious and genetic diseases, and technological progress in PCR-based testing platforms, leading to improved accuracy and faster turnaround times in both clinical and research settings
- Furthermore, growing demand for high-throughput, cost-effective, and multiplex testing solutions is accelerating the uptake of Polymerase Chain Reaction (PCR) Multiplex Assays, thereby significantly boosting the industry's growth
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Analysis
- Polymerase Chain Reaction (PCR) Multiplex Assays, offering simultaneous detection of multiple genetic targets in a single reaction, are increasingly vital components of modern molecular diagnostics and research workflows in both clinical and laboratory settings due to their high sensitivity, specificity, and rapid turnaround time
- The escalating demand for PCR multiplex assays is primarily fueled by the growing adoption of advanced molecular diagnostics, rising prevalence of infectious diseases and genetic disorders, and the need for faster, cost-effective testing solutions
- U.K. dominated the polymerase chain reaction (PCR) multiplex assays market with the largest revenue share of 42% in 2025, characterized by advanced healthcare infrastructure, increasing investment in molecular diagnostics, and a strong presence of key industry players, with the U.K. experiencing substantial growth in PCR testing installations, particularly in hospitals, diagnostic laboratories, and research institutes, driven by innovations in multiplex assay panels and automated PCR platforms
- Germany is expected to be the fastest growing region in the polymerase chain reaction (PCR) multiplex assays market during the forecast period, projected to expand at a CAGR of 12%, due to increasing government initiatives for early disease detection, rising adoption of high-throughput PCR systems, and expanding research and development activities in molecular diagnostics and biotechnology
- The clinical diagnostics segment accounted for the largest market revenue share of 52.3% in 2025, supported by the rising prevalence of infectious diseases, genetic disorders, and demand for rapid pathogen detection
Report Scope and Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Segmentation
|
Attributes |
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Trends
“Rising Adoption of Multiplex Testing for Infectious Diseases”
- A prominent and accelerating trend in the Europe PCR multiplex assays market is the increasing adoption of multiplexing technologies, which allow simultaneous detection of multiple pathogens or genetic targets in a single assay. This trend is being driven by the need for faster, more comprehensive diagnostic testing across clinical and research laboratories
- For instance, laboratories are increasingly employing multiplex PCR assays to detect respiratory infections, sexually transmitted infections, and viral load monitoring in a single run, reducing turnaround time and improving diagnostic efficiency
- Multiplex PCR also reduces reagent consumption and operational costs, making it an attractive option for large-scale clinical diagnostics, especially in high-throughput testing scenarios such as hospital laboratories and reference centers
- Healthcare providers are leveraging multiplex PCR for outbreak surveillance and pathogen identification, allowing timely treatment decisions and more effective infection control strategies
- In addition, the development of user-friendly multiplex platforms with automated sample processing is enhancing adoption in both hospital laboratories and point-of-care settings, supporting faster and reliable results
- The integration of advanced chemistries, high-resolution detection systems, and refined primer-probe designs further improves assay sensitivity and specificity, driving broader adoption in research and clinical applications
- Multiplex PCR assays are increasingly replacing singleplex assays in routine diagnostic workflows, enabling laboratories to manage multiple targets efficiently and reduce human error. Regulatory approvals for multiplex panels targeting infectious diseases, oncology biomarkers, and genetic disorders have accelerated their clinical use across Europe
- Academic research institutions are utilizing multiplex PCR to study gene expression patterns, microbial communities, and rare mutations, boosting translational research outputs
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Dynamics
Driver
“Increasing Prevalence of Infectious and Genetic Diseases”
- The rising prevalence of infectious diseases, including influenza, COVID-19, HIV, and multi-drug resistant pathogens, is a key driver for the Europe PCR Multiplex Assays market. Multiplex PCR provides rapid and reliable pathogen detection critical for timely intervention
- Similarly, the increasing incidence of genetic disorders, rare diseases, and cancers requiring molecular diagnostics fuels the demand for high-precision PCR assays
- For instance, hospital laboratories and diagnostic centers are adopting multiplex panels for simultaneous testing of hereditary conditions, oncology markers, and prenatal screening target
- The growing need for comprehensive testing during pandemic and epidemic scenarios has further emphasized the importance of multiplex PCR for efficient laboratory workflows
- In addition, reimbursement policies and government initiatives supporting molecular diagnostics have encouraged laboratories to invest in multiplex platforms
- Awareness campaigns among clinicians regarding early detection and personalized treatment approaches are driving greater utilization of multiplex PCR assays
- The increasing number of research studies and clinical trials relying on multiplex PCR for biomarker discovery, infection surveillance, and pharmacogenomics applications further boosts market demand
- Hospitals and reference laboratories are upgrading diagnostic infrastructure to meet rising patient volumes, emphasizing multiplex PCR for high-throughput testing. Investment in laboratory automation and integration with laboratory information systems (LIS) also facilitates broader adoption of multiplex assays
Restraint/Challenge
“High Costs and Complex Workflow Limiting Adoption”
- Despite the clear advantages, the Europe PCR Multiplex Assays market faces challenges due to the high costs associated with multiplex platforms and reagents, which may hinder adoption in smaller laboratories or budget-constrained regions
- For instance, a 2023 study in Germany highlighted that certain respiratory pathogen multiplex panels missed rare viral strains, prompting laboratories to continue using singleplex confirmatory tests alongside multiplex assays
- The need for skilled personnel to perform multiplex PCR, interpret results, and maintain assay accuracy adds to operational complexity and may slow adoption
- Regulatory compliance and validation requirements for multiplex panels are stringent, requiring extensive quality control and proficiency testing, which increases time-to-market for new assays
- In addition, variations in sensitivity and specificity across different multiplex panels for the same targets can create challenges in standardization and result interpretation
- Smaller laboratories may be hesitant to invest in multiplex platforms due to initial capital expenditure, ongoing maintenance costs, and the need for dedicated laboratory space
- High reagent costs and supply chain issues for primers, probes, and consumables can further increase the overall cost per test. For instance, in 2022, several European diagnostic centers reported delayed procurement of multiplex PCR kits due to global primer shortages, impacting testing schedules
- Operational challenges such as cross-contamination risk, pipetting errors, and instrument calibration issues may impact assay reliability
- Integration with existing laboratory workflows and information systems can be complex, requiring additional training and infrastructure upgrades
- Some multiplex PCR assays may have limitations in target coverage or flexibility, leading laboratories to maintain complementary singleplex assays, adding operational burden
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Scope
The market is segmented on the basis of products and services, applications, and end users.
• By Products and Services
On the basis of products and services, the Europe PCR Multiplex Assays market is segmented into reagents and consumables, instruments and accessories, and software and services. The reagents and consumables segment dominated the market with the largest revenue share of 46.5% in 2025, driven by the high recurring demand for primers, probes, enzymes, and other critical assay components. Reagents are essential for assay performance, influencing sensitivity, specificity, and reproducibility. This segment benefits from frequent repeat orders and the rapid expansion of molecular testing across hospitals and diagnostic laboratories. Increasing adoption of multiplex panels, standardized kits, and automated workflows further supports growth. Key manufacturers, including Thermo Fisher Scientific and Qiagen, continuously launch optimized reagent kits, enhancing reliability and workflow efficiency. The segment also benefits from regulatory approvals, local distribution networks, and competitive pricing. The rising prevalence of infectious diseases, genetic disorders, and routine molecular testing drives sustained demand. Additionally, the growing emphasis on laboratory efficiency and quality control ensures long-term market stability. Expansion in clinical and research applications adds to segment dominance.
The software and services segment is expected to witness the fastest CAGR of 9.8% from 2026 to 2033. Its growth is driven by increasing demand for integrated data analysis platforms, cloud-based reporting, and automated laboratory solutions. Enhanced software enables accurate multiplex assay interpretation, reduces human error, and ensures compliance with regulatory standards. The growth is also supported by the adoption of laboratory information management systems (LIMS), advanced analytics, and remote monitoring solutions. Pharmaceutical and biotechnology companies increasingly rely on software for assay design, validation, and reporting. Cloud-based services facilitate secure data storage, collaboration across research teams, and efficient workflow management. Academic institutions and research centers are adopting software platforms to accelerate genomic research and biomarker identification. Automation in clinical and R&D labs further fuels adoption. As laboratories move toward high-throughput screening and digital integration, this segment is projected to grow rapidly.
• By Applications
On the basis of applications, the market is segmented into clinical diagnostics and research & development. The clinical diagnostics segment accounted for the largest market revenue share of 52.3% in 2025, supported by the rising prevalence of infectious diseases, genetic disorders, and demand for rapid pathogen detection. Multiplex PCR offers faster turnaround times, high sensitivity, and the ability to detect multiple targets simultaneously. Hospitals and diagnostic labs increasingly integrate these assays into routine testing for respiratory, gastrointestinal, and sexually transmitted infection panels. Growing healthcare expenditure, improved reimbursement policies, and adoption of molecular diagnostics in primary care further strengthen this segment. The segment benefits from ongoing technological advancements, regulatory approvals, and increased laboratory automation. Standardized diagnostic kits ensure reproducibility and scalability. Clinical diagnostics also support disease surveillance and outbreak management, driving consistent adoption. High patient volumes and increasing molecular testing requirements maintain the dominance of this segment.
The research & development segment is anticipated to witness the fastest CAGR of 10.1% from 2026 to 2033, driven by rising investments in genomics, drug discovery, and biomarker development. Pharmaceutical and academic researchers increasingly use multiplex PCR for high-throughput screening and validation studies. The need for rapid, multiplexed testing in preclinical and translational research accelerates adoption. Expansion of personalized medicine initiatives and molecular-targeted therapies fuels demand. Research labs require flexible platforms capable of handling multiple assays simultaneously. Growing collaborations between biotechnology companies and research institutions enhance market opportunities. Automation and digital integration improve workflow efficiency and data accuracy. Advanced assay kits and customizable panels further drive adoption. The segment benefits from expanding funding for genomics and molecular biology research. Increasing focus on early disease detection and biomarker validation supports rapid growth.
• By End Users
On the basis of end users, the market is segmented into hospitals, clinical laboratories, pharmaceutical and biotechnology companies, research institutes, and others. The hospitals segment dominated with a market share of 44.7% in 2025, driven by increasing patient volumes, rising adoption of multiplex PCR for rapid diagnostics, and integration into routine testing workflows. Hospitals are investing in advanced molecular platforms to ensure timely clinical decision-making, outbreak monitoring, and accurate detection of pathogens. Government support, reimbursement initiatives, and the growing importance of precision medicine contribute to segment dominance. Hospitals increasingly focus on efficiency, high-throughput testing, and standardization of diagnostic protocols. Availability of trained personnel, access to advanced equipment, and adherence to quality standards enhance adoption. Expansion in both public and private hospital networks further strengthens market share. Clinical need for rapid pathogen detection in emergency and critical care units sustains growth. Continuous improvement in assay sensitivity, specificity, and workflow integration reinforces hospital adoption.
The pharmaceutical and biotechnology companies segment is expected to witness the fastest CAGR of 10.3% from 2026 to 2033, driven by increasing utilization of multiplex PCR for drug discovery, preclinical research, and biomarker identification. Rising focus on personalized medicine and molecular-targeted therapies is accelerating adoption. Multiplex assays allow efficient screening of multiple genetic targets in a single test, saving time and resources. Biotechnology firms leverage these assays for high-throughput screening and validation studies. Investment in R&D, collaborations with academic institutions, and expansion of molecular research facilities support growth. Automation, digital data analysis, and integrated laboratory workflows further drive segment adoption. Regulatory requirements for data accuracy and reproducibility enhance the reliance on multiplex assays. Increasing demand for novel therapeutics and diagnostic solutions continues to propel the segment. Growth in emerging biotech hubs across Europe contributes to rapid expansion.
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Regional Analysis
- The Europe polymerase chain reaction (PCR) multiplex assays market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing government initiatives for early disease detection, rising investment in molecular diagnostics, and expanding research and development activities in biotechnology and molecular diagnostics
- Healthcare institutions and research organizations in the region are increasingly adopting high-throughput PCR platforms and multiplex assay panels for faster, accurate, and cost-effective testing
- This widespread adoption is further supported by advanced healthcare infrastructure, growing awareness about early disease diagnosis, and the strong presence of key industry players, establishing PCR multiplex assays as a preferred solution across clinical, diagnostic, and research applications
U.K. Polymerase Chain Reaction (PCR) Multiplex Assays Market Insight
The U.K. polymerase chain reaction (PCR) multiplex assays market captured the largest revenue share of 42% in 2025 within Europe, fueled by the expansion of PCR testing installations in hospitals, diagnostic laboratories, and research institutes. Increasing investment in multiplex assay panels and automated PCR platforms, combined with strong government support and advanced healthcare infrastructure, is further driving market growth.
Germany Polymerase Chain Reaction (PCR) Multiplex Assays Market Insight
The Germany polymerase chain reaction (PCR) multiplex assays market is expected to grow at the fastest CAGR of 12% during the forecast period, driven by rising adoption of high-throughput PCR systems, expanding molecular diagnostics research, and government initiatives promoting early disease detection. The country’s emphasis on innovation, coupled with increasing R&D activities in biotechnology, is propelling the market across hospitals, clinical laboratories, and research centers.
Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market Share
The Polymerase Chain Reaction (PCR) Multiplex Assays industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific (U.S.)
- Agilent Technologies (U.S.)
- Roche Diagnostics (Switzerland)
- Sigma-Aldrich (U.S.)
- Illumina (U.S.)
- Bio-Rad Laboratories (U.S.)
- Merck KGaA (Germany)
- PerkinElmer (U.S.)
- Takara Bio (Japan)
- BD Biosciences (U.S.)
- Pfizer (U.S.)
- GenScript (China)
- Novogene (China)
- Promega Corporation (U.S.)
- MGI Tech (China)
- Seqirus (Australia)
- Agendia (Netherlands)
- Eurofins Scientific (Luxembourg)
- Hologic (U.S.)
Latest Developments in Europe Polymerase Chain Reaction (PCR) Multiplex Assays Market
- In December 2021, Eurofins Technologies launched a CE‑marked multiplex RT‑PCR respiratory panel capable of simultaneously detecting and differentiating SARS‑CoV-2, Influenza A, Influenza B, RSV A, and RSV B in a single test. This development enabled clinical laboratories to perform faster, more comprehensive respiratory pathogen screening, reducing turnaround time and improving patient management during the pandemic
- In October 2022, Novacyt introduced its genesig SARS-CoV-2 Winterplex 3G assay, a multiplex PCR panel designed to detect SARS-CoV-2, Influenza A & B, and RSV in one assay. This product received regulatory clearance in the UK, providing hospitals and diagnostic labs with an efficient solution for high-throughput respiratory testing, particularly during peak flu seasons
- In April 2023, Roche Diagnostics unveiled its LightCycler PRO System, a next-generation multiplex qPCR platform supporting over 200 modular LightMix assays. The system is engineered for high-throughput applications, allowing simultaneous detection of multiple pathogens or genetic targets, which significantly improves laboratory efficiency and diagnostic accuracy for complex clinical testing workflows
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.