Market Analysis and Insights Europe Point of Care Testing (POCT) Device Market
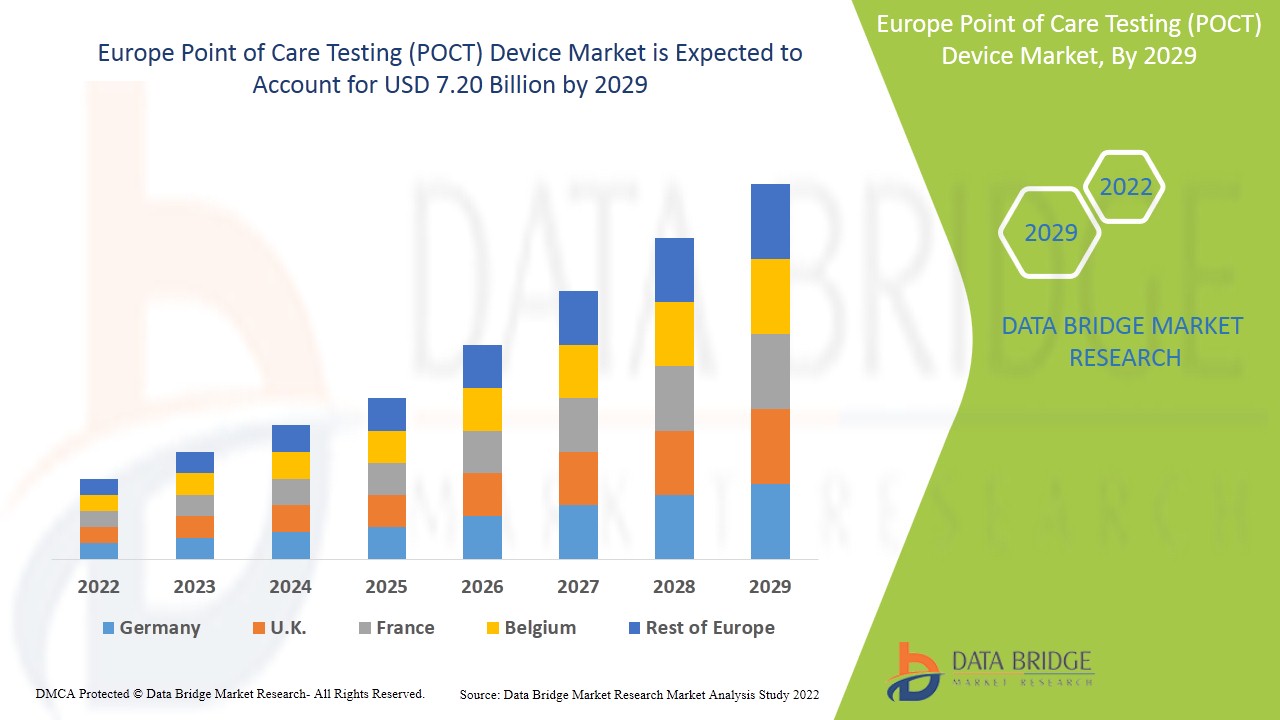
Data Bridge Market Research analyses that the point of care testing (POCT) device market growing at a CAGR of 9.3% in the forecast period of 2022-2029 and would reach to an estimated value of 7.20 billion by the forecast period of 2022 to 2029..
A point-of-care testing (POCT) diagnostic device is a device that is used to collect specific clinical data from patients in a clinical setting. As infection identification leads to decreased antibiotic intake, fewer laboratory tests for patients, and lower treatment costs, point-of-care testing (POCT) is rapidly emerging into a preferred test option. This is not only beneficial to patients, but it is also beneficial to hospitals, settings with restricted resources
One of the primary factors driving the expansion of the point-of-care testing (POCT) market is the increase in funding from various sources around the world. Increased demand for point-of-care tests due to divisions such as operating rooms, cath labs, ICU, emergency room, and neonatal intensive care units, among others, in order to deliver effective and speedy patient treatment accelerates the point-of-care testing (POCT) market expansion.
The surge in demand for home healthcare is driving manufacturers to develop user-friendly portable devices, and the introduction of smartphone-based healthcare solutions, digital technologies, and embedded vision-based solutions are all having an impact on the point-of-care testing (POCT) market. Furthermore, the growing importance of point-of-care diagnostics in environmental monitoring and public health, as well as the growing number of CLIA-waived point of care tests, technological advancements, and an increase in healthcare expenditure, all has a positive impact on the point-of-care testing (POCT) market.
However, high product costs, pricing pressure due to reimbursement cuts and budget constraints, and strict regulatory rules are projected to hinder the expansion of the point-of-care testing (POCT) market.
This point of care testing (POCT) device market report provides details of new recent developments, trade regulations, import export analysis, production analysis, value chain optimization, market share, impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on point of care testing (POCT) device market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Europe Point of Care Testing (POCT) Device Market Scope and Market Size
Europe point of care testing (POCT) device market is segmented on the basis of product type, prescription mode, distribution channel and end users. The growth amongst these segments will help you analyse meagre growth segments in the industries, and provide the users with valuable market overview and market insight s to help them in making strategic decisions for identification of core market applications.
- Based on product type, the Europe point of care testing (POCT) device market is segmented into blood glucose testing kits, cardiometabolic monitoring kits, infectious disease testing kits, cholesterol testing kits, pregnancy and fertility tests kits, tumor/cancer markers, urinalysis testing kits, cholesterol test strips, hematology testing kits, drugs of abuse testing kits, fecal occult testing kits, rapid coagulation testing kits, and others. Cardiometabolic monitoring kits are further segmented into cardiac markers, blood gas/electrolytes testing kits, HbA1c testing kits, and lipids testing. Also, infectious disease testing kits are further segmented into influenza testing kits, HIV testing kits, hepatitis C testing kits, sexually-transmitted diseases testing kits, tropical diseases testing kits, healthcare-associated infections and respiratory infections testing kit. Cholesterol testing kits are further divided into prothrombin time testing kits and activated clotting time testing kit.
- On the basis of prescription mode, the Europe point of care testing (POCT) device market is segmented into prescription based testing and over-the-counter (OTC) testing.
- On the basis of distribution channel, the Europe point of care testing (POCT) device market is segmented into direct tenders and retail.
- On the basis of end users, the Europe point of care testing (POCT) device market is segmented into hospital, clinics, ambulatory care, home healthcare, and research laboratory. Hospital segment is further categorized into emergency department High product costs, pricing pressure due to reimbursement cuts and budget constraints, and strict regulatory rules are projected to hinder the expansion of the point-of-care testing (POCT) market.
Point of Care Testing (POCT) Device Market Country Level Analysis
The point of care testing (POCT) device market is analysed and market size insights and trends are provided by product type, prescription mode, distribution channel, end users as referenced above.
The countries covered in the point of care testing (POCT) device market report are Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, and Rest of Europe.
The country section of the point of care testing (POCT) device market report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as consumption volumes, production sites and volumes, import export analysis, price trend analysis, cost of raw materials, down-stream and upstream value chain analysis are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The point of care testing (POCT) device market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for point of care testing (POCT) device market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the point of care testing (POCT) device market. The data is available for historic period 2010 to 2020.
Competitive Landscape and Point of Care Testing (POCT) Device Market Share Analysis
The point of care testing (POCT) device market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to point of care testing (POCT) device market.
Some of the major players operating in the point of care testing (POCT) device market are Abbott., Abaxis, PTS Diagnostics, F. Hoffmann-La Roche Ltd, Samsung Medison Co., Ltd., Bio-Rad Laboratories, Inc., The Menarini Group, Nova Biomedical, AccuBioTech Co., Ltd., BD, Chembio Diagnostics, Inc., Danaher.,EKF DIAGNOSTICS HOLDINGS PLC, Johnson & Johnson Services, Inc., Quidel Corporation, Sekisui Diagnostics, Siemens, Trinity Biotech Ireland, Shubh Surgical & Pharmaceuticals and Sarita Surgical Works among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.