Europe Prescription Digital Therapeutics Dtx Market
Market Size in USD Million
CAGR :
% 
 USD
179.50 Million
USD
846.80 Million
2022
2030
USD
179.50 Million
USD
846.80 Million
2022
2030
| 2023 –2030 | |
| USD 179.50 Million | |
| USD 846.80 Million | |
|
|
|
|
Europe Prescription Digital Therapeutics (PDTx) Market Analysis and Size
Prescription digital therapeutics have been developed as a boon for a number of diseases such as substance use disorder (SUD), ADHD, major depressive disorder (MDD), opioid use disorder (OUD), insomnia, epilepsy, multiple sclerosis, autism spectrum disorder, and cardiovascular disease. Patients with several unmet requirements may find prescription digital therapies to be the most vital aspect of their lives. Presently, numerous research & clinical studies are going on which is anticipated to create a competitive advantage for several manufacturers to develop new and innovative technology.
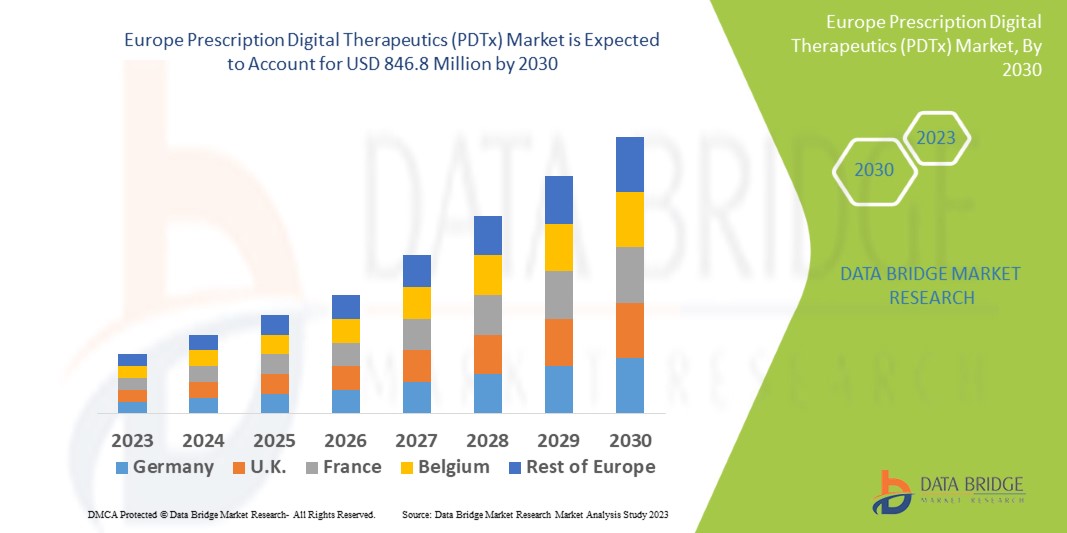
Data Bridge Market Research analyses a growth rate in the prescription digital therapeutics (PDTx) market in the forecast period 2023-2030. The expected CAGR of prescription digital therapeutics (PDTx) market is tend to be around 21.4% in the mentioned forecast period. The market value is USD 179.5 million in 2022, and it would grow upto USD 846.8 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Prescription Digital Therapeutics (PDTx) Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Mechanism (Input Mechanisms, Output Mechanisms), Category (Medication Augmentation, Medication Replacement), Treatment (Outpatient Treatment, Monotherapy), Software (Software For Respiratory Conditions, Software For Mental Health, Software For Opioid Use Disorder, Software For Diabetes, Others), Services (Behavioral Microservices, Medical Microservices), App Accessibility (Android, iOS, Windows), App Type (Native Apps, Web Apps), Application (Substance Use Disorder (SUD), Opioid Use Disorder (OUD), Attention Deficit/Hyperactivity Disorder (ADHD), Alzheimer’s Disease, Major Depressive Disorder (MDD), Insomnia, Epilepsy, Movement Disorder, Multiple Sclerosis, Migraine, Autism Spectrum Disorder, Oncology, Inflammation, Respiratory, Cardiovascular, Pain Management, Metabolic Conditions, Others), Patients (Children, Adults) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
ResMed (U.S.), SAMSUNGHEALTHCARE (South Korea), Biofourmis (U.S.), Novartis AG (Switzerland), Medtronic (Ireland), Pear Therapeutics, Inc. (U.S.), Voluntis (France), Omada Health, Inc. (U.S.), GAIA AG (Germany), Welldoc’s Bluestar (U.S.), Solera Network (U.S.), Akili Interactive Labs, Inc. (U.S.), Better Therapeutics, LLC (U.S.), BigHealth (U.S.), Biofourmis (U.S.), Click Therapeutics, Inc. (U.S.), Happify, Inc. (U.S.), Limbix Health, Inc. (U.S.), Naturalcycles Nordic AB (Sweden), NuvoAir AB (Sweden), Sensyne Health plc. (U.K.), Xealth (U.S.) |
|
Market Opportunities |
|
Market Definition
Prescription digital therapies is a kind of software that is used to treat numerous ailments. Artificial intelligence, diverse algorithms, and virtual reality are widely used to develop these therapeutic instruments. Patients sometimes use prescription digital therapeutics software and services to get any form of treatment they needed it. These medicines are tightly regulated by the FDA and other regulatory authorities and can only be used with an appropriate prescription from a doctor.
Europe Prescription Digital Therapeutics (PDTx) Market Dynamics
Drivers
- Increasing incidence of Chronic Diseases
The increasing prevalence of chronic diseases is a primary factor boosting the prescription digital therapeutics (PDTx) market's growth rate during the forecast period. According to the World Health Organization (WHO), around 463 million adults will have diabetes in 2020. In 2021, 35.2% of the European population aged 16 years and above reported a long-term illness. The number of people who are surviving with cancer has also been growing. At present, 16–17 million European citizens are expected to either be in the treatment phase for cancer or are in post-treatment phase, and this number will also increase significantly over the next 10–20 years. Therefore, this factor increases the growth of the market.
- Developing Pipeline for Potential Products
A major pipeline of potential items estimated to be released throughout the forecast period is expected to benefit the growth of prescription digital therapeutics (PDTx). For instance, Pear-011 (anxiety GAD), Pear-015 (depression MDD), which are used in the treatment of adults suffering from chronic insomnia and depression, and CT-155 (schizophrenia), CT-152 (major depressive disorder), which are used in the treatment of adults with depression and schizophrenia, are products of potential pear therapeutics clinical therapeutics products in phase III. This factor improves the market growth.
Opportunities
- Increased Research Activities
The research activities associated with the prescription digital therapeutics is increasing and thus boosting the growth of the marketthus boosting the market. For instance, NuvoAir AB was certified as class Im medical device in August 2020. The company has achieved this certification due to its advanced features involving certified bluetooth spirometer, combined with the healthcare portal and connected patient app. This certification has improved the brand value among the physicians and patients. Thus, this factor created much opportunity for the market growth.
- Growing Adoption of Software for Diabetes
The software for diabetes is anticipated to increase the market as the world is having major population suffering from the diabetes and is the primary cause of death. As per a survey, 463 million populations suffering from diabetes and this date is growing continuously at a rapid rate, because of this, Europe health expenditure on diabetes is also increasing and multiple research is going on to find the new and easy way to treat the patients. Therefore, this factor helps to create much opportunities for the growth of the market.
Restraints/Challenges
- Security and Data Management
PDTx transmits information over the internet, hence the risk of unauthorized access and manipulation of these solutions could threaten product confidence and patient care. The FDA has issued guidance on cybersecurity, labelling, and documentation for pre-marketing submissions, but several agencies do not currently require pre-market medical device safety audits to be completed. It is the complete responsibility of the device manufacturers to define the level of cybersecurity risk related with their products and perform actions to reduce this risk. This impedes the market growth.
- Difficulty in Adaptation of Digital Therapeutics by Doctors
Not all doctors and providers are not familiar with the increasing digitization. Doctors and healthcare professionals should get familiar with digital therapy solutions before using their mass adoption. They should analyze the results of clinical trials before recommending the solutions to their patients. To perform this, few DTx companies opt for pharmaceutical model wherein partnerships with distributors are used to inform doctors about clinical trial results, new solutions, and decisions. Thus, this hampers the market growth.
This prescription digital therapeutics (PDTx) market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the prescription digital therapeutics (PDTx) market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In 2020, Biofourmis announced that its Biovitals platform had been widely used by the Ministry of Health (MOH) in Singapore to observe the health for COVID-19 patients. This initiative enabled the nurses and clinicians to diagnose the disease symptoms early, enabling patients to achieve effective treatment. Thus, this initiative improved the company’s revenue by increasing the adoption rate during COVID-19 pandemic.
Europe Prescription Digital Therapeutics (PDTx) Market Scope
The prescription digital therapeutics (PDTx) market is segmented on the basis of mechanism, category, treatment, software, services, app accessibility, app type, application, patients. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Mechanism
- Input Mechanisms
- Output Mechanisms
Category
- Medication Augmentation
- Medication Replacement
Treatment
- Outpatient Treatment
- Monotherapy
Software
- Software for Respiratory Conditions
- Software for Mental Health
- Software for Opioid Use Disorder
- Software for Diabetes
- Others
Services
- Behavioral Microservices
- Medical Microservices
App Accessibility
- Android
- iOS
- Windows
App Type
- Native Apps
- Web Apps
Application
- Substance Use Disorder (SUD)
- Opioid Use Disorder (OUD)
- Attention Deficit/Hyperactivity Disorder (ADHD)
- Alzheimer’s Disease
- Major Depressive Disorder (MDD)
- Insomnia
- Epilepsy
- Movement Disorder
- Multiple Sclerosis
- Migraine
- Autism Spectrum Disorder
- Oncology
- Inflammation
- Respiratory
- Cardiovascular
- Pain Management
- Metabolic Conditions
- Others
Patients
- Children
- Adults
Prescription Digital Therapeutics (PDTx) Market Regional Analysis/Insights
The prescription digital therapeutics (PDTx) market is analyzed and market size insights and trends are provided by mechanism, category, treatment, software, services, app accessibility, app type, application, patients as referenced above.
The major countries covered in the prescription digital therapeutics (PDTx) market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
Germany is anticipated to grow with the highest CAGR in the forecast period as the application of prescription digital therapeutics is increasing across several European countries with technological advancement in software. Furthermore, the increasing number of domestic companies operating in the market is also boosting the market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Prescription Digital Therapeutics (PDTx) Market Share Analysis
The prescription digital therapeutics (PDTx) market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Prescription digital therapeutics (PDTx) market
Key players operating in the prescription digital therapeutics (PDTx) market include:
- ResMed (U.S.)
- SAMSUNGHEALTHCARE (South Korea)
- Biofourmis (US)
- Novartis AG (Switzerland)
- Medtronic (Ireland)
- Pear Therapeutics, Inc. (U.S.)
- Voluntis (France)
- Omada Health, Inc. (U.S.)
- GAIA AG (Germany)
- Welldoc’s Bluestar (U.S.)
- Solera Network (U.S.)
- Akili Interactive Labs, Inc. (U.S.)
- Better Therapeutics, LLC (U.S.)
- BigHealth (U.S.)
- Biofourmis (U.S.)
- Click Therapeutics, Inc. (U.S.)
- Happify, Inc. (U.S.)
- Limbix Health, Inc. (U.S.)
- Naturalcycles Nordic AB (Sweden)
- NuvoAir AB (Sweden)
- Sensyne Health plc. (U.K.)
- Xealth (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.