Europe Vaccines Market
Market Size in USD Billion
CAGR :
% 
 USD
48.18 Billion
USD
79.74 Billion
2024
2032
USD
48.18 Billion
USD
79.74 Billion
2024
2032
| 2025 –2032 | |
| USD 48.18 Billion | |
| USD 79.74 Billion | |
|
|
|
|
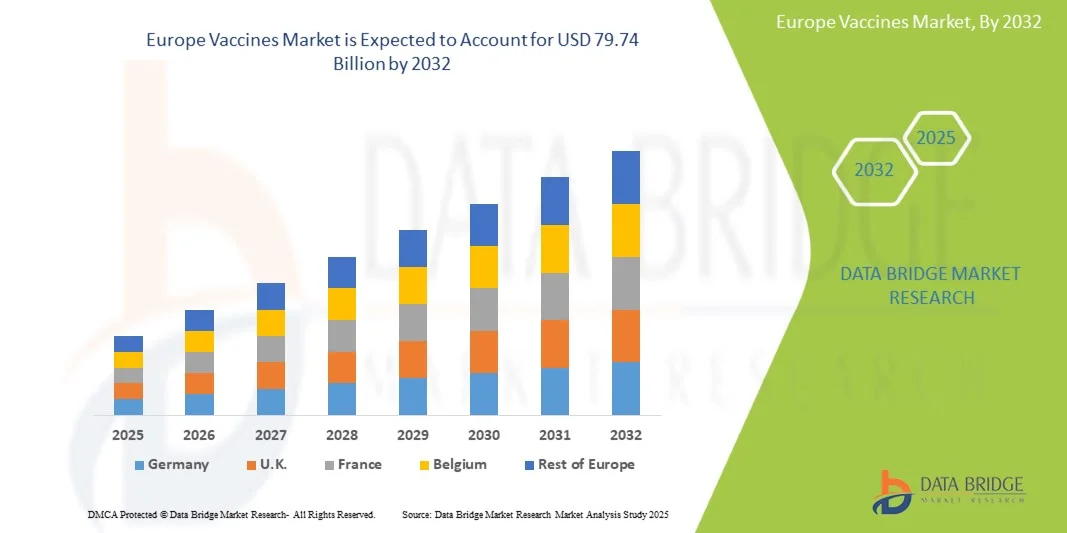
Europe Vaccines Market Size
- The Europe Vaccines Market size was valued at USD 48.18 billion in 2024 and is expected to reach USD 79.74 billion by 2032, at a CAGR of 6.50% during the forecast period
- The market growth is largely fueled by increasing awareness of infectious diseases, rising government and private investments in healthcare infrastructure, and ongoing research and development in vaccine technologies
- Furthermore, growing demand for preventive healthcare measures, expansion of immunization programs, and the development of novel vaccines for emerging and re-emerging diseases are accelerating the uptake of vaccines, thereby significantly boosting the industry's growth
Europe Vaccines Market Analysis
- Vaccines, designed to provide active immunity against infectious diseases, are increasingly vital components of global healthcare systems in both pediatric and adult populations due to their proven efficacy, safety, and role in preventing widespread outbreaks
- The escalating demand for vaccines is primarily fueled by increasing public awareness of immunization, rising prevalence of infectious diseases, government vaccination programs, and growing focus on preventive healthcare globally
- Germany dominated the Europe Vaccines Market with the largest revenue share of 28.7% in 2024, supported by advanced healthcare infrastructure, early adoption of new vaccines, strong R&D investment, and active participation in clinical trials and immunization programs
- The U.K. is expected to be the fastest-growing country in Europe Vaccines Market during the forecast period, with a projected CAGR of 7.2%, driven by expanding vaccination initiatives, increasing public awareness, growth of specialty clinics, and improved healthcare access
- The injectable vaccines segment dominated the Europe Vaccines Market in 2024 with a revenue share of 70%, owing to its wide application in routine, recommended, and required immunization programs
Report Scope and Europe Vaccines Market Segmentation
|
Attributes |
Europe Vaccines Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Vaccines Market Trends
“Enhanced Convenience Through Technological and Logistical Advancements”
- A significant and accelerating trend in the Europe Vaccines Market is the implementation of advanced technologies and streamlined logistics to improve vaccine distribution, storage, and administration. Digital tracking systems, cold chain monitoring, and integration with electronic health records enhance operational efficiency and ensure vaccines reach target populations safely
- For instance, in 2023, Pfizer implemented smart cold chain monitoring across its European distribution centers to track the temperature and location of COVID-19 vaccine shipments in real-time
- These innovations enable healthcare providers to schedule vaccinations accurately, monitor inventory in real-time, and reduce wastage caused by improper storage or transport delays. For instance, BioNTech partnered with DHL in 2022 to use IoT-enabled containers for safe transport of mRNA vaccines across Europe
- Automation in large-scale vaccination programs has facilitated faster distribution, better patient scheduling, and improved coverage rates for critical vaccines. For instance, Spain introduced automated appointment scheduling systems for influenza vaccines in 2024 to optimize clinic workflows
- Technological advancements in vaccine formulation and stabilization support longer shelf life, enabling broader distribution across regions with varying infrastructure capabilities. In 2021, Moderna announced improved formulation techniques allowing its mRNA vaccines to be stored at standard refrigeration temperatures
- Governments and private healthcare providers are adopting these measures to reduce administrative burdens, improve patient compliance, and ensure equitable access to vaccines. For example, France’s national immunization program leveraged digital health portals in 2022 to monitor and remind citizens about vaccinations
- This trend toward digitalized, efficient vaccine management is transforming public health strategies and strengthening immunization programs across Europe
Europe Vaccines Market Dynamics
Driver
“Growing Demand Due to Increased Immunization Programs and Awareness”
- The rising prevalence of infectious diseases, combined with government-led immunization initiatives, is driving the Europe Vaccines Market. For instance, the European Commission’s 2023 initiative to expand influenza vaccination coverage among seniors increased vaccine demand significantly.
- Public education campaigns and awareness programs are encouraging higher uptake, especially among children, elderly populations, and immunocompromised individuals. In 2022, the UK’s “Vaccines for Life” campaign led to a notable increase in pediatric vaccination rates
- Improvements in cold chain logistics and vaccine tracking have enhanced reliability, accessibility, and efficiency
- For instance, DHL’s use of smart temperature-monitoring containers for COVID-19 vaccines in Germany ensured safe deliveries in 2023
- Expansion of healthcare infrastructure, including vaccination centers and mobile units, allows wider reach in urban and rural areas. Spain’s mobile vaccination units in 2024 provided access to remote communities
- Collaborations between pharmaceutical companies, healthcare providers, and government agencies ensure timely vaccine supply and administration. Pfizer-BioNTech’s joint EU distribution program in 2021 is a prime instance
- Research into combination vaccines and novel delivery methods has improved efficacy, safety, and patient compliance. For instance, GSK launched a new quadrivalent influenza vaccine in 2022
- International travel and imported infections highlight the importance of robust vaccination programs. Italy increased traveler-focused vaccination campaigns in 2023 following imported measles cases
- Incentive programs, reimbursement schemes, and public-private partnerships improve affordability and access. Germany implemented partial reimbursement for high-risk adult vaccines in 2022
- Hospitals, clinics, and community health centers are serving as key distribution points, strengthening adoption. The NHS in the UK expanded local clinic vaccination capacity in 2023
- Increased disease awareness, better healthcare infrastructure, and efficient vaccine delivery systems are expected to continue fueling market growth in Europe
Restraint/Challenge
“High Costs, Supply Chain Constraints, and Regulatory Hurdles”
- High production and procurement costs for innovative vaccines can limit accessibility in price-sensitive regions
- For instance, early procurement of mRNA COVID-19 vaccines in 2021 faced budgetary challenges for smaller EU countries
- Supply chain disruptions, including cold chain failures, raw material shortages, and transport delays, may affect timely vaccine delivery. In 2022, delayed shipments of influenza vaccines were reported in Poland due to packaging material shortages
- Stringent regulatory requirements, such as clinical trials and documentation, can delay approvals and market entry. For instance, EMA’s extended review of new RSV vaccines in 2023 slowed their rollout
- Vaccine hesitancy and misinformation in certain population groups may reduce uptake despite awareness campaigns. France reported lower-than-expected measles vaccination rates in 2022 due to hesitancy
- Limited healthcare workforce in some areas can slow distribution and administration, impacting coverage rates. Italy’s rural regions faced shortages of trained vaccinators in 2023
- Budget constraints and reimbursement complexities in public health systems may restrict large-scale vaccine procurement. In 2021, smaller EU nations delayed adult hepatitis vaccination programs due to funding limitations
- Seasonal demand fluctuations, particularly for influenza vaccines, require careful inventory management to avoid shortages or wastage. Germany reported stock imbalances during the 2022 flu season
- Coordination among manufacturers, healthcare providers, logistics companies, and government agencies is complex and can impact efficiency. Poland experienced delays in COVID-19 vaccine distribution due to interagency coordination issues in 2021
- Sudden emergence of new pathogens may require rapid scaling of vaccine production, creating logistical and financial challenges. The 2022 monkeypox outbreak in Spain highlighted these challenges
- Intellectual property, patent regulations, and licensing agreements can affect vaccine availability in specific countries. EU-wide licensing negotiations delayed rollout of certain combination vaccines in 2023
- Overcoming these challenges requires investment in manufacturing capacity, robust supply chain systems, public education, and regulatory harmonization to ensure consistent vaccine access across Europe
Europe Vaccines Market Scope
The market is segmented on the basis of composition, type, kind, age of administration, diseases, route of administration, end user, and distribution channel.
• By Composition
On the basis of composition, the Europe Vaccines Market is segmented into combination vaccines and mono vaccines. The combination vaccines segment dominated the market with a revenue share of 52% in 2024, driven by increased preference for vaccines that protect against multiple diseases simultaneously, reducing the number of injections required. Governments and healthcare providers encourage combination vaccines to improve immunization compliance and coverage. For instance, the MMRV (Measles, Mumps, Rubella, and Varicella) vaccine is widely used in pediatric programs across Germany and France. The segment benefits from higher acceptance among parents and healthcare professionals due to reduced clinic visits and improved patient adherence. Increasing research and development in combination vaccines, supported by collaborations between pharmaceutical companies and public health agencies, is strengthening market dominance. Rising awareness about childhood vaccination schedules and government-led immunization programs are further boosting adoption. Furthermore, combination vaccines reduce healthcare costs by minimizing the total number of vaccine doses administered. Enhanced formulation techniques ensure efficacy and safety, which improves overall trust in combination immunization programs. The segment is supported by robust supply chains and cold chain logistics, ensuring timely delivery and storage. Efforts to expand combination vaccine coverage in rural and semi-urban regions are also contributing to the high market share. Manufacturing innovations and patent protection mechanisms help maintain premium pricing while supporting sustained demand. The increasing incidence of preventable diseases across Europe reinforces the necessity of combination vaccines, making them the backbone of immunization strategies.
The mono vaccines segment is expected to witness the fastest CAGR of 9.8% from 2025 to 2032, driven by targeted disease prevention campaigns and the growing need for specialized vaccines. Countries like Italy and Spain are expanding mono-vaccine programs for influenza, typhoid, and meningococcal diseases. Mono vaccines allow flexibility in immunization schedules and are critical for adults and high-risk populations. Emerging biotech companies are developing next-generation mono vaccines with enhanced efficacy and fewer side effects. The rise in epidemic outbreaks, seasonal diseases, and traveler vaccination programs is fueling demand. Public awareness campaigns and increasing physician recommendations are further supporting uptake. Governments are offering funding and subsidy schemes for mono vaccines to ensure higher coverage rates. Mono vaccines are increasingly being integrated into workplace vaccination programs for healthcare workers and at-risk populations. Clinical trial programs and early access initiatives accelerate the introduction of new mono vaccines. The segment benefits from advanced cold chain logistics and digital monitoring of vaccine distribution. Increasing demand from specialty clinics and hospitals reinforces market growth. Overall, the mono vaccine segment is projected to expand rapidly due to its adaptability and targeted protection.
• By Type
On the basis of type, the Europe Vaccines Market is segmented into subunit, recombinant, polysaccharide and conjugate vaccines, live-attenuated vaccines, inactivated vaccines, toxoid vaccines, and DNA vaccines. The subunit, recombinant, polysaccharide, and conjugate vaccines segment dominated the market in 2024 with a revenue share of 48%, attributed to their high efficacy, safety profile, and adaptability for multiple diseases such as pneumococcal infections, meningococcal disease, and hepatitis. These vaccines are widely adopted in pediatric and adult immunization programs. The segment benefits from increasing government vaccination initiatives and recommendations by organizations like WHO and ECDC. Advanced manufacturing technologies and rigorous quality controls have enhanced public confidence. Subunit and conjugate vaccines are crucial in reducing disease burden among vulnerable populations, including children and immunocompromised individuals. Multi-dose formulations reduce clinic visits and healthcare costs, strengthening market adoption. Continuous R&D in novel adjuvants and delivery mechanisms improves immunogenicity. Collaborations between biotech firms and national health authorities facilitate widespread distribution. Effective cold chain infrastructure ensures potency and accessibility across Europe. Educational campaigns targeting caregivers, physicians, and community health workers further boost uptake. Strategic partnerships for vaccine rollouts in urban and rural areas maintain high coverage levels. Market expansion is reinforced by increasing disease prevalence and the need for routine immunization against multiple pathogens.
The live-attenuated vaccines segment is projected to witness the fastest CAGR of 8.7% from 2025 to 2032, driven by rising adoption for measles, mumps, rubella, varicella, and other viral diseases. Countries such as France, Germany, and the UK are expanding immunization schedules to include newer live-attenuated formulations. Improved safety profiles and efficacy data encourage higher uptake among children and adults. Live-attenuated vaccines are increasingly used in combination programs to reduce the number of injections. Expanding pediatric immunization programs and school-based vaccination drives boost growth. Advanced storage technologies improve cold chain stability, ensuring vaccine effectiveness. Increasing international travel and cross-border disease risks promote live-attenuated vaccine demand. Government funding and reimbursement schemes support accessibility. Partnerships between vaccine manufacturers and healthcare providers ensure timely distribution. Public awareness campaigns highlighting long-term immunity benefits contribute to acceptance. Ongoing research into novel viral strains and improved formulations strengthens the segment. The segment’s growth is also supported by WHO and EU immunization guidelines advocating live vaccines for outbreak prevention.
• By Kind
On the basis of kind, the Europe Vaccines Market is segmented into routine vaccine, recommended vaccine, and required vaccine. The routine vaccine segment dominated the market with a 55% revenue share in 2024, as it encompasses vaccines included in standard immunization schedules across countries. Pediatric programs, school entry requirements, and public health campaigns ensure high coverage rates. Vaccines for DPT, polio, and MMR are widely administered, providing consistent demand. Governments implement subsidy programs to ensure affordability, particularly for low-income households. The segment benefits from established distribution networks and cold chain infrastructure. Healthcare providers and clinics actively monitor vaccination coverage. Educational initiatives improve caregiver compliance and awareness. International guidelines and WHO recommendations reinforce routine vaccine adoption. Consistent efficacy and safety data support continued preference. Pediatric-focused campaigns and integration into national health records maintain high uptake. Policy mandates and early-age administration drive demand across Europe. Routine vaccines represent the foundation of public immunization strategy, ensuring the prevention of common childhood diseases.
The recommended vaccine segment is expected to witness the fastest CAGR of 8.2% from 2025 to 2032, fueled by adult immunization programs for influenza, hepatitis, and travel-related vaccines. Awareness campaigns targeting seniors, healthcare workers, and frequent travelers are driving uptake. Increasing outbreaks of seasonal influenza and emerging infectious diseases create urgency. Government programs encourage high-risk population coverage. Partnerships with pharmacies, clinics, and occupational health providers enhance accessibility. Data-driven tracking systems support timely administration. Innovative adjuvants improve immune response, enhancing trust and compliance. The segment benefits from increased insurance coverage and reimbursement. Mobile vaccination units and online scheduling platforms improve reach. Travel vaccination campaigns in countries like Spain and Italy promote rapid adoption. Physician recommendations strongly influence uptake. Recommended vaccines support preventive healthcare initiatives and reduce the burden on hospitals and emergency care.
• By Age of Administration
On the basis of age of administration, the Europe Vaccines Market is segmented into pediatric vaccines and adult vaccines. The pediatric vaccine segment dominated the market in 2024 with a revenue share of 60%, driven by widespread immunization programs targeting infants and children. Vaccines for diseases such as DPT, MMR, and pneumococcal infections are administered through national immunization schedules in countries like Germany, France, and the UK. Government initiatives and school-entry requirements support high vaccination coverage. Pediatric vaccines reduce disease burden and prevent outbreaks, enhancing public health outcomes. Early-age vaccination ensures long-term immunity and lowers healthcare costs over a lifetime. Continuous R&D in safe and effective pediatric formulations strengthens market confidence. Educational campaigns aimed at parents and caregivers improve adherence. Pediatric vaccines benefit from robust cold chain logistics and well-established distribution channels. National health authorities actively monitor compliance and coverage rates. Public-private partnerships facilitate access to underserved populations. Innovative delivery mechanisms, such as combination vaccines, improve compliance. Pediatric vaccination programs remain a cornerstone of Europe’s public health strategy.
The adult vaccine segment is projected to witness the fastest CAGR of 9% from 2025 to 2032, driven by increasing awareness of adult immunization needs for influenza, hepatitis, and travel vaccines. Countries like Italy, Spain, and the UK are expanding adult vaccination programs to cover high-risk populations. Workplace vaccination campaigns and travel clinics are key adoption drivers. Physician recommendations and government awareness campaigns improve uptake among seniors and immunocompromised individuals. Novel vaccine formulations with improved efficacy and reduced side effects encourage acceptance. Adult vaccination supports preventive healthcare and reduces hospitalizations during outbreaks. Insurance coverage and reimbursement policies enhance accessibility. Online appointment booking systems and mobile vaccination units improve reach and convenience. Partnerships between hospitals, clinics, and pharmacies ensure efficient distribution. Public health campaigns emphasize the importance of booster doses and seasonal vaccines. Increasing incidence of infectious diseases among adults further fuels demand. Adult vaccines are gaining strategic importance as Europe focuses on lifelong immunization programs.
• By Diseases
On the basis of diseases, the Europe Vaccines Market is segmented into pneumococcal disease, measles, mumps & varicella, DPT, hepatitis, influenza, typhoid, meningococcal, rabies, Japanese encephalitis, yellow fever, and others. The pneumococcal disease segment dominated the market with a 38% revenue share in 2024, due to high prevalence among infants, young children, and elderly populations. Pneumococcal conjugate vaccines (PCV) are included in routine immunization schedules across Germany, France, and the UK. Government funding and public awareness campaigns have boosted uptake. Vaccination reduces hospitalizations and healthcare costs associated with pneumonia and related infections. Advanced vaccine formulations enhance safety and immunogenicity. Pediatric and elderly vaccination programs are strongly supported by healthcare providers. National vaccination coverage targets reinforce adoption. Cold chain infrastructure ensures vaccine potency. Collaboration between vaccine manufacturers and public health agencies strengthens distribution. Educational initiatives increase caregiver and patient compliance. Widespread use of PCV reduces antibiotic resistance by preventing bacterial infections. Pneumococcal vaccination is a key driver for disease prevention programs.
The influenza segment is expected to witness the fastest CAGR of 9.5% from 2025 to 2032, driven by seasonal vaccination campaigns, increased awareness of flu-related complications, and higher vaccination rates among elderly and high-risk populations. Governments in Europe implement annual influenza immunization programs targeting seniors, healthcare workers, and children. Vaccination reduces morbidity, mortality, and hospital burden during flu seasons. Emerging vaccine technologies, such as quadrivalent and high-dose formulations, enhance efficacy. Collaboration with pharmacies and community clinics ensures broader reach. Awareness campaigns and digital scheduling platforms simplify vaccination processes. Insurance coverage and reimbursement policies improve affordability. Physician recommendations play a crucial role in increasing uptake. Urban and rural outreach programs expand coverage in under-served areas. Increasing prevalence of influenza-associated complications during winter months reinforces demand. Seasonal influenza vaccines support overall preventive healthcare strategies. Continuous R&D into next-generation flu vaccines ensures market growth.
• By Route of Administration
On the basis of route of administration, the Europe Vaccines Market is segmented into injectable, oral, and nasal vaccines. The injectable vaccines segment dominated the market in 2024 with a revenue share of 70%, owing to its wide application in routine, recommended, and required immunization programs. Injectable vaccines offer proven efficacy, long-term immunity, and compatibility with multiple vaccine types including subunit, conjugate, and live-attenuated vaccines. Pediatric, adult, and travel vaccination programs rely predominantly on injectable formulations. Hospitals, clinics, and specialty centers are the primary administration points. Advanced syringe and needle technologies reduce discomfort and improve compliance. Regulatory approvals, safety data, and standardized protocols reinforce adoption. Cold chain logistics ensure potency and safe delivery. Injectable vaccines are essential in reducing disease burden and preventing outbreaks. Public health campaigns educate populations on injection safety and importance. Combination injectable vaccines minimize clinic visits. National immunization policies strongly favor injectable vaccines for core programs. Injectable vaccines are the backbone of Europe’s vaccination strategy.
The oral vaccines segment is projected to witness the fastest CAGR of 8.7% from 2025 to 2032, driven by ease of administration, patient compliance, and growing adoption in pediatric and travel vaccination programs. Oral polio vaccines and rotavirus vaccines are key examples with increasing acceptance. Countries like France and Italy have integrated oral vaccines in public health campaigns. Vaccine delivery without needles reduces fear and improves adherence, especially among children. Oral vaccines simplify mass immunization in schools and community settings. Innovative formulations and stabilization techniques enhance efficacy. Distribution through pharmacies and clinics improves accessibility. Public awareness campaigns highlight convenience and safety. International travel programs encourage oral vaccines for travelers. Partnerships between healthcare providers and manufacturers ensure supply chain efficiency. Increasing demand for non-invasive vaccines drives growth. Oral vaccines are becoming critical in expanding preventive healthcare coverage.
• By End User
On the basis of end user, the Europe Vaccines Market is segmented into community hospitals, hospitals, specialty centers, clinics, and others. The hospitals segment dominated the market in 2024 with a revenue share of 50%, supported by their ability to provide comprehensive immunization services for pediatric, adult, and specialty vaccines. Hospitals have trained staff, cold chain facilities, and digital record-keeping systems. Government programs often supply vaccines directly to hospitals to ensure high coverage. Hospitals are central to disease surveillance and outbreak management. High patient footfall and accessibility reinforce dominance. Hospitals administer routine, recommended, and required vaccines efficiently. Continuous training and awareness campaigns improve staff adherence to immunization protocols. Collaboration with public health agencies strengthens outreach efforts. Hospital-based vaccination drives reach high-risk and underserved populations. Hospitals ensure regulatory compliance and monitor adverse events. Partnerships with pharmaceutical companies facilitate vaccine supply and innovation.
The specialty centers segment is expected to witness the fastest CAGR of 9% from 2025 to 2032, driven by increased adoption of specialized vaccines for rare diseases, travel immunization, and high-risk populations. Specialty centers offer focused care, expertise in immunization protocols, and tailored patient counseling. Centers provide vaccines not commonly available in general hospitals or clinics. Collaboration with research institutions accelerates access to innovative vaccines. Patient education and compliance programs support uptake. Specialty centers are critical for adult and high-risk population vaccination programs. Government grants and private funding enhance operational capabilities. Expansion of multispecialty networks improves regional access. Partnerships with insurance providers improve affordability. Digital platforms facilitate appointment scheduling and reminders. Specialty centers ensure safe handling of vaccines and manage cold chain logistics. Rising demand for personalized vaccination programs strengthens growth prospects.
• By Distribution Channel
On the basis of distribution channel, the Europe Vaccines Market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market in 2024 with a revenue share of 45%, as hospitals remain the primary distribution point for routine and specialty vaccines. Hospital pharmacies provide vaccines directly to patients under healthcare supervision, ensuring adherence to immunization schedules. They maintain cold chain storage, manage inventory, and track vaccine administration. Hospital pharmacies collaborate with government programs to ensure widespread availability. The segment benefits from robust healthcare infrastructure and high patient trust. Vaccines distributed through hospital pharmacies reach pediatric, adult, and high-risk populations. Centralized record-keeping supports disease surveillance. Hospitals coordinate with clinics and specialty centers to maintain supply consistency. Educational programs improve awareness and adherence. Hospital pharmacies also support clinical trials and research initiatives. Partnerships with vaccine manufacturers secure timely supply. High-quality control standards reinforce dominance.
The online pharmacy segment is expected to witness the fastest CAGR of 10% from 2025 to 2032, driven by increasing e-commerce adoption, convenience, and broader vaccine accessibility. Online platforms enable patients to order vaccines for delivery or schedule pick-up at nearby pharmacies or clinics. Digital health records and reminders improve compliance. Telemedicine integration allows consultation prior to vaccination. Online pharmacies are increasingly offering vaccines for travel, seasonal influenza, and specialized immunization programs. Government initiatives support e-health platforms for wider access. Convenience, reduced waiting time, and contactless ordering drive growth. Online pharmacies cater to urban and semi-urban populations. Partnerships with logistics providers ensure cold chain maintenance during transit. Mobile apps and web portals enhance patient engagement. Insurance integration enables reimbursement for vaccines purchased online. Digital marketing campaigns raise awareness of available vaccines.
Europe Vaccines Market Regional Analysis
- Germany dominated the Europe Vaccines Market with the largest revenue share of 28.7% in 2024, supported by advanced healthcare infrastructure, early adoption of new vaccines, strong R&D investment, and active participation in clinical trials and immunization programs
- The U.K. is expected to be the fastest-growing country in Europe Vaccines Market during the forecast period, with a projected CAGR of 7.2%, driven by expanding vaccination initiatives, increasing public awareness, growth of specialty clinics, and improved healthcare access
- The market is further supported by robust research and development activities, early adoption of new vaccine technologies, and the presence of leading pharmaceutical companies actively participating in clinical trials and immunization programs
Germany Europe Vaccines Market Insight
Germany vaccines dominated the Europe Vaccines Market with the largest revenue share of 28.7% in 2024, supported by advanced healthcare infrastructure, early adoption of new vaccines, strong R&D investment, and active participation in clinical trials and immunization programs. Hospitals, specialty centers, and clinics are key contributors to market growth, with both routine and recommended vaccines being widely administered across pediatric and adult populations.
U.K. Europe Vaccines Market Insight
The U.K. vaccines is expected to be the fastest-growing country in Europe for the Europe Vaccines Market during the forecast period, with a projected CAGR of 7.2%, driven by expanding vaccination initiatives, increasing public awareness, growth of specialty clinics, and improved healthcare access. Government-led immunization programs, coupled with private healthcare efforts, are enhancing vaccine penetration and fueling market adoption in both urban and rural areas.
Europe Vaccines Market Share
The Vaccines industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Moderna (U.S.)
- Johnson & Johnson and its afiliates (U.S.)
- GSK Plc. (U.K.)
- Sanofi (France)
- AstraZeneca (U.K.)
- Novavax (U.S.)
- Bharat Biotech (India)
- Serum Institute of India (India)
- SINOVAC (China)
- China National Pharmaceutical Group Co Ltd. (China)
- Merck & Co., Inc. (U.S.)
- CSL Limited (Australia)
- BioNTech SE(Germany)
- CureVac SE (Germany)
Latest Developments in Europe Vaccines Market
- In September 2025, the European Commission announced it was conducting unannounced inspections at the premises of an unnamed company operating in the vaccine sector. These inspections are part of an antitrust investigation stemming from concerns that the company may have breached EU rules by abusing a dominant market position. Specifically, the Commission is probing potential exclusionary practices, which could amount to anticompetitive disparagement. National competition authorities joined European Commission officials during the raids to support the investigation
- In September 2025, Moderna's UK general manager, Darius Hughes, defended the country's pharmaceutical pricing environment amid industry criticism, as the company opened a £150 million vaccine manufacturing site in Oxfordshire. This facility, capable of producing up to 100 million mRNA vaccine doses annually (scalable to 250 million in a pandemic), is part of a £1 billion, decade-long strategic partnership with the UK government. Despite other pharmaceutical giants like MSD, AstraZeneca, and Eli Lilly curtailing UK investment over pricing concerns, Hughes emphasized Moderna's ongoing commitment to the UK, particularly in pandemic preparedness and vaccine innovation
- In May 2025, the European Medicines Agency (EMA) recommended Valneva's single-shot vaccine for chikungunya virus, making it the first preventive shot to potentially gain approval in Europe. Valneva's VLA1553 vaccine had previously been approved in the U.S. in November, where it is sold under the brand name Ixchiq. During late-stage trials, the vaccine showed high efficacy, inducing neutralizing antibody levels in 98.9% of participants within 28 days of vaccination
- In February 2025, Bavarian Nordic's chikungunya vaccine, Vimkunya, received marketing authorization from the European Medicines Agency (EMA). This vaccine, intended for individuals aged 12 years and older, represents a significant advancement in preventing the chikungunya disease in Europe
- In January 2025, the EU-funded VACCELERATE project concluded, marking the completion of a groundbreaking pan-European initiative. Over its duration, VACCELERATE significantly contributed to the advancement of vaccine research and clinical trial coordination across Europe. Through collaboration, innovation, and strategic infrastructure development, the project established a lasting impact on pandemic preparedness and public health
- In May 2023, GSK entered into a €1.4 billion licensing agreement with CureVac to develop vaccines for flu, Covid-19, and avian flu. The UK pharmaceutical company agreed to pay €400 million upfront, with potential additional milestone payments. GSK will take over the full development of these vaccines, which may include combination vaccines for flu and Covid-19, positioning it to compete with mRNA vaccine leaders like Moderna and Pfizer
- In May 2023, Stablepharma and partner BB-NCIPD Ltd (Bul Bio) signed a second exclusive supply agreement to develop a fridge-free Tetanus mono vaccine. This collaboration aims to produce a vaccine that does not require refrigeration, enhancing vaccine accessibility in low-resource settings
- In February 2023, Bharat Biotech International Limited (BBIL), based in Hyderabad, in-licensed GSK’s investigational Shigella vaccine, altSonflex1-2-3. This vaccine targets Shigellosis, a serious bacterial diarrhoeal disease primarily affecting children under five in low- and middle-income countries. The vaccine was previously tested in Phase I and II trials by GSK in Europe and Africa, showing a strong immune response and a favorable safety profile. Bharat Biotech will now handle Phase III trials, regulatory processes, and large-scale production
- In February 2022, the European Medicines Agency (EMA) recommended the approval of Valneva's COVID-19 vaccine, VLA2001, for use in the European Union. This inactivated vaccine was developed by Valneva SE in collaboration with Dynavax Technologies. It was authorized for medical use in the European Union in June 2022
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.