Global 3rd Generation Lentiviral Vector Market
Market Size in USD Million
CAGR :
% 
 USD
162.31 Million
USD
476.20 Million
2024
2032
USD
162.31 Million
USD
476.20 Million
2024
2032
| 2025 –2032 | |
| USD 162.31 Million | |
| USD 476.20 Million | |
|
|
|
|
Global 3rd Generation Lentiviral Vector Market Analysis
The global 3rd generation lentiviral vector market is experiencing robust growth, primarily driven by the increasing demand for advanced gene therapies and personalized treatment options for genetic disorders, cancers, and chronic diseases. 3rd generation lentiviral vectors are considered the most advanced class of viral vectors, offering further enhancements in safety, efficiency, and reduced immunogenicity compared to previous generations. These vectors are used to deliver therapeutic genes into patient cells in various applications, such as ex vivo gene therapies, cancer immunotherapies, and treatments for inherited genetic disorders like hemophilia, sickle cell disease, and beta-thalassemia.
The growth of the market is further fueled by the rapid advances in molecular biology and biotechnology, which enable researchers to develop more efficient and targeted gene therapies. In addition, the rising prevalence of genetic disorders, the expansion of oncology applications, and increased investments in gene therapy research are propelling the market for 3rd generation lentiviral vectors. With the continued success of clinical trials and the increasing approval of gene therapy products utilizing lentiviral vectors, the market is poised for further expansion. However, challenges such as high production costs, complexity in scaling manufacturing processes, and regulatory hurdles may slow the adoption of these vectors, especially in emerging markets.
Global 3rd Generation Lentiviral Vector Market Size
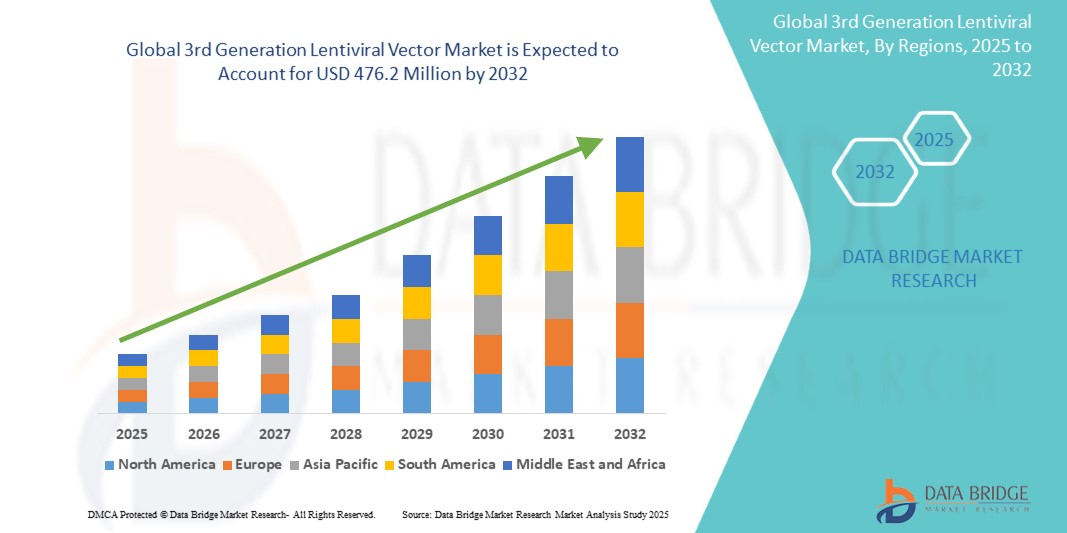
Global 3rd generation lentiviral vector market size was valued at USD 162.31 million in 2024 and is projected to reach USD 476.2 million by 2032, with a CAGR of 14.40% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Global 3rd Generation Lentiviral Vector Market Trends
“Advancements in Gene Therapy Applications”
A key trend in the 3rd generation lentiviral vector market is the continuous evolution and expansion of gene therapy applications. 3rd generation lentiviral vectors are increasingly being used for a range of therapeutic purposes, including gene editing, cancer immunotherapy, and the treatment of genetic disorders. These vectors have become essential tools in the development of advanced therapies like CRISPR-based gene editing, where they serve as carriers to deliver genome-editing components into target cells. The ability of 3rd generation lentiviral vectors to transduce both dividing and non-dividing cells with high efficiency makes them ideal for gene therapy applications, especially in treating a wide variety of diseases.
Cancer immunotherapy is another rapidly growing application area for 3rd generation lentiviral vectors. The vectors are used to genetically modify immune cells, such as T-cells, to better recognize and attack cancer cells. The growing success of CAR-T (Chimeric Antigen Receptor T-cell) therapies and the increasing number of ongoing clinical trials are expected to drive the demand for lentiviral vectors in the oncology sector. The ability to deliver genetic material with precision and safety is also expanding the potential uses of 3rd generation lentiviral vectors in treating inherited and rare genetic disorders, where traditional therapies have shown limited efficacy. As these therapies progress, the role of 3rd generation lentiviral vectors will continue to evolve, becoming a critical enabler of advanced medicine.
Global Digital Genome Market Segmentation
|
Attributes |
Global 3rd Generation Lentiviral Vector Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Merck KGaA(Germany), Lonza(Switzerland), FUJIFILM Diosynth Biotechnologies U.S.A. Inc.( U.S.), Cobra Biologics Ltd.(U.K.), Brammer Bio(U.S.), Waisman Biomanufacturing, Genezen(U.S.), YPOSKESI(France), Advanced BioScience(U.S.), Laboratories Inc. (ABL Inc.)(U.S.), Novasep Holding S.A.S(France), ATVIO Biotech Ltd(U.K.) and others. |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Global 3rd Generation Lentiviral Vector Market Definition
A 3rd Generation Lentiviral Vector is an earlier, widely used system for gene delivery, offering improved safety and performance compared to 1st and 2nd generation systems. These vectors are used in both research and therapeutic applications to deliver genetic material into target cells. The design of 3rd generation lentiviral vectors emphasizes safety, efficiency, and versatility.
Global 3rd Generation Lentiviral Vector Market
Drivers
- Increasing Demand for Gene Therapy
The global rise in the demand for gene therapies is a major driver for the growth of the third-generation lentiviral vector market. Lentiviral vectors, particularly the third-generation ones, are essential tools in gene therapy due to their ability to deliver therapeutic genes into target cells with high efficiency and stable gene expression. This is particularly important for treating genetic disorders, cancers, and other conditions that were previously difficult to manage with traditional therapies. The increasing number of clinical trials and FDA-approved gene therapy products using lentiviral vectors highlights the growing importance of this technology in modern medicine. As more rare and genetic diseases are being targeted with gene-based solutions, the demand for advanced and safer viral vector systems like third-generation lentiviral vectors continues to rise.
- Improved Safety and Efficacy of 3rd Generation Lentiviral Vectors
The third-generation lentiviral vectors offer significant improvements over earlier versions in terms of safety and efficacy, making them increasingly popular for gene delivery applications. Third-generation vectors are engineered to reduce the risk of insertional mutagenesis (where the inserted genetic material disrupts the host genome) and improve the precision of gene integration, addressing one of the major concerns with older vector systems. These improvements, such as the removal of viral genes that could cause harmful effects, have made third-generation lentiviral vectors more attractive for clinical and therapeutic applications. As researchers and clinicians continue to seek safer and more efficient methods for gene therapy, the use of third-generation lentiviral vectors is expected to expand, driving market growth.
Opportunities
- Expansion of Applications in Oncology and Genetic Disorders
One of the most significant opportunities for the third-generation lentiviral vector market is the growing application of these vectors in oncology and genetic disorders. Lentiviral vectors are increasingly being utilized for the development of gene-based cancer immunotherapies, where they are used to deliver genes that enhance the immune system’s ability to target and destroy cancer cells. In addition, for genetic disorders like sickle cell anemia, hemophilia, and inherited blindness, third-generation lentiviral vectors offer promising solutions. As more genetic diseases are understood, gene therapy using these vectors becomes a viable treatment option, creating substantial growth opportunities. The market is likely to see continued expansion as the technology advances, with more successful clinical outcomes leading to broader acceptance and adoption of lentiviral vector-based therapies.
- Growing Support from Government and Private Sector for Gene Therapy Research
Another major opportunity for the lentiviral vector market is the increasing support from both governments and private sector investors for gene therapy research and development. Governments in regions such as the U.S. and Europe are offering grants and funding for the development of gene therapies, recognizing their potential to address previously untreatable diseases. Private sector companies, including biotech firms and pharmaceutical companies, are also investing heavily in gene therapy as part of their pipeline for novel treatments. This growing financial support enables more research into third-generation lentiviral vectors, further advancing the technology and increasing its adoption. With more resources dedicated to improving gene therapy outcomes, the market for third-generation lentiviral vectors is likely to see strong growth in the coming years.
Restraints/Challenges
- High Production Costs and Complexity
One of the major challenges in the 3rd generation lentiviral vector market is the high production costs and complexity involved in manufacturing these vectors. Producing lentiviral vectors involves intricate processes, including the generation of viral particles, transfection of producer cells, and purification, which can be time-consuming and expensive. The cost of producing high-quality lentiviral vectors with the desired levels of purity and infectivity is significant. As a result, the high cost of goods sold (COGS) could limit the accessibility of lentiviral vector-based therapies, especially in low- and middle-income countries where healthcare budgets are more constrained. In addition, the complexity of manufacturing processes may lead to variability in production and challenges in scaling up production for clinical or commercial use.
- Regulatory Hurdles and Long Approval Timelines
Regulatory challenges and lengthy approval processes present another restraint for the market. Gene therapies using lentiviral vectors require rigorous clinical trials and regulatory scrutiny before they can reach the market. Regulatory bodies like the FDA and EMA demand extensive safety and efficacy data before granting approval for gene therapy products. As gene therapies are relatively new, there are no well-established guidelines for these products, and regulatory agencies must develop new frameworks to assess their safety and effectiveness. The long approval timelines and regulatory hurdles can delay the commercial availability of gene therapy products, thereby affecting market growth. Furthermore, ensuring compliance with evolving regulatory standards, especially in different regions, can be challenging for companies developing lentiviral vector-based therapies.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global 3rd Generation Lentiviral Vector Market Scope
The market is segmented by product type, application, and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Pre-packaged Vectors,
- Custom Vectors
Application
- Gene Therapy,
- Cancer Immunotherapy,
- Genetic Disorders,
- HIV Treatment
End-User
- Biopharmaceutical Companies,
- Academic & Research Institutions,
- Contract Development and Manufacturing Organizations
Global 3rd Generation Lentiviral Vector Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product type, application, and end user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America dominates the global 3rd generation lentiviral vector market, with the United States being a key contributor due to its advanced healthcare infrastructure, significant research investments, and established regulatory frameworks. The U.S. is home to numerous biotech companies and research institutions focusing on the development and application of gene therapies, which is driving the demand for lentiviral vectors. The market is further boosted by the presence of key market players, as well as the active involvement of regulatory bodies such as the FDA in facilitating the approval of gene therapies.
The Asia-Pacific region is the fastest growing market for third-generation lentiviral vectors, driven by rapid advancements in biotechnology and an expanding healthcare infrastructure. Countries like China, Japan, India, and South Korea are increasingly investing in biotechnology research and development, leading to a surge in clinical trials and the application of gene therapies. The growing focus on healthcare modernization and the rising prevalence of genetic disorders and cancer in the region further stimulate the demand for gene-based treatments, thus boosting the adoption of lentiviral vectors.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Global 3rd Generation Lentiviral Vector Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Global 3rd Generation Lentiviral Vector Market Leaders Operating in the Market Are:
- Merck KGaA(Germany)
- Lonza (Switzerland)
- FUJIFILM Diosynth Biotechnologies U.S.A. Inc. ( U.S.)
- Cobra Biologics Ltd. (U.K.)
- Brammer Bio (U.S.)
- Waisman Biomanufacturing, Genezen (U.S.)
- YPOSKESI (France)
- Advanced BioScience (U.S.)
- Laboratories Inc. (ABL Inc.) (U.S.)
- Novasep Holding S.A.S (France)
- ATVIO Biotech Ltd (U.K.)
Latest Developments in Global 3rd Generation Lentiviral Vector Market
- In June 2022, Avid Bioservices, Inc. opened its analytical and process development (AD/PD) suites. These suites were part of the company's lentiviral vector development and Current Good Manufacturing Plant (CGMP) manufacturing facility
- In May 2022, AGC Biologics said that it would add viral vector suspension technology and space to its commercial-grade site in Longmont, Colorado. This would make it easier to study and make gene therapies
- In September 2024, Rentschler Biopharma has introduced a new lentiviral vector manufacturing toolbox designed to enhance the production of advanced therapies. This innovative solution aims to support the growing demand for gene and cell therapies, offering improved scalability, efficiency, and safety in the manufacturing of lentiviral vectors for therapeutic applications
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.