Global Acid Sphingomyelinase Deficiency Drugs Market
Market Size in USD Million
CAGR :
% 
 USD
151.70 Million
USD
397.61 Million
2024
2032
USD
151.70 Million
USD
397.61 Million
2024
2032
| 2025 –2032 | |
| USD 151.70 Million | |
| USD 397.61 Million | |
|
|
|
|
Acid Sphingomyelinase Deficiency Drugs Market Size
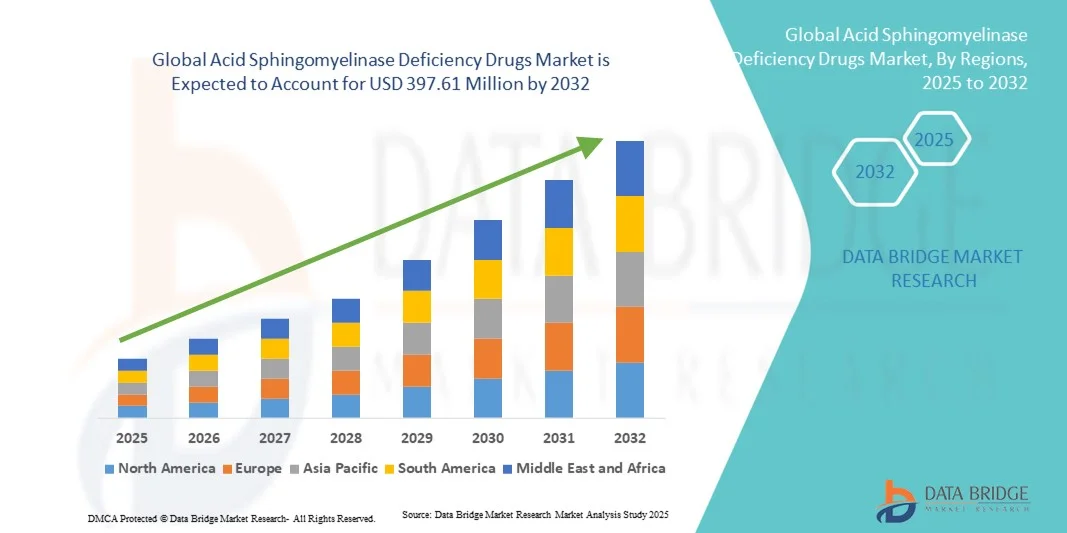
- The global acid sphingomyelinase deficiency drugs market size was valued at USD 151.70 Million in 2024 and is expected to reach USD 397.61 Million by 2032, at a CAGR of 12.80% during the forecast period
- The market growth is largely fueled by the increasing prevalence of acid sphingomyelinase deficiency (ASMD), rising awareness of rare genetic disorders, and advancements in targeted enzyme replacement therapies
- Furthermore, growing investment in research and development, expanding healthcare infrastructure, and increasing availability of innovative treatment options are accelerating the adoption of ASMD drugs, thereby significantly boosting the industry's growth
Acid Sphingomyelinase Deficiency Drugs Market Analysis
- Acid sphingomyelinase deficiency drugs, offering targeted therapeutic solutions for lysosomal storage disorders, are increasingly vital components of modern treatment protocols in both pediatric and adult patients due to their efficacy, safety profile, and clinical adoption
- The escalating demand for acid sphingomyelinase deficiency drugs is primarily driven by increasing prevalence of lysosomal storage disorders, rising awareness among healthcare professionals, and advancements in therapeutic options
- The North America region dominated the acid sphingomyelinase deficiency drugs market with the largest revenue share of 45.2% in 2024, characterized by advanced healthcare infrastructure, high R&D investments, early adoption of novel therapies, and the strong presence of key pharmaceutical companies. The U.S. specifically experienced substantial growth due to active participation in clinical trials, accelerated drug approvals, and rising physician and patient awareness regarding treatment options. Early access programs and government initiatives further facilitated uptake, making North America the leading regional market
- Asia-Pacific is expected to be the fastest-growing region in the acid sphingomyelinase deficiency drugs market during the forecast period, with a projected CAGR of 8.3% from 2025 to 2032, fueled by increasing healthcare expenditure, improved access to diagnostic facilities, and rising awareness among physicians and patients. Countries such as China, Japan, and India are witnessing accelerated adoption due to expanding hospital networks, government-led rare disease programs, and partnerships with multinational pharmaceutical companies
- The Olipudase Alfa segment dominated the global acid sphingomyelinase deficiency drugs market with the largest market revenue share of 46.3% in 2024, owing to its well-established therapeutic efficacy in reducing sphingomyelin accumulation and mitigating disease progression
Report Scope and Acid Sphingomyelinase Deficiency Drugs Market Segmentation
|
Attributes |
Acid Sphingomyelinase Deficiency Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Acid Sphingomyelinase Deficiency Drugs Market Trends
Rising Focus on Targeted and Innovative Therapies
- A significant and accelerating trend in the global acid sphingomyelinase deficiency drugs market is the growing development and adoption of enzyme replacement therapies (ERTs) and gene therapies, which aim to provide targeted treatment for patients with ASMD. This focus on innovative therapies is enhancing clinical outcomes and patient quality of life
- For instance, olipudase alfa, an enzyme replacement therapy developed by Sanofi, has shown promising results in improving lipid metabolism and reducing spleen and liver size in clinical trials, representing a major breakthrough for patients with ASMD
- The increasing prevalence of rare genetic disorders and improved diagnostic capabilities are enabling early identification and intervention, which is driving the demand for specialized treatment options
- Pharmaceutical companies are investing in advanced research platforms to explore novel molecules and delivery methods, ensuring more efficient and safe treatment outcomes
- The market is witnessing collaborations between biotech firms, academic institutions, and healthcare providers to accelerate clinical development and regulatory approvals, further driving innovation
- There is also a growing emphasis on patient-centric solutions, including personalized dosing regimens, improved safety profiles, and therapies that target multiple disease manifestations, supporting long-term disease management
Acid Sphingomyelinase Deficiency Drugs Market Dynamics
Driver
Growing Need Due to Rising Rare Disease Awareness and Therapeutic Advancements
- The increasing awareness of rare lysosomal storage disorders such as ASMD, combined with the advancement of enzyme replacement therapies and gene therapy approaches, is a significant driver for market growth
- For instance, in January 2023, Sanofi announced positive Phase III trial results for olipudase alfa in patients with non-neurological ASMD, demonstrating significant improvements in spleen volume reduction and lipid profiles. Such developments are expected to increase physician adoption and patient access globally
- As healthcare systems increasingly focus on rare disease management, there is a growing push to provide innovative therapies for previously untreatable conditions, fueling market demand
- Advances in genetic testing and diagnostic tools are enabling earlier detection, which increases patient eligibility for targeted therapies
- Government initiatives and rare disease frameworks, particularly in regions such as North America and Europe, are supporting the development, approval, and reimbursement of ASMD therapies, further bolstering market growth
- Expanded patient support programs and awareness campaigns by leading pharmaceutical companies are facilitating better disease understanding, increasing therapy uptake across hospitals and specialty clinics
Restraint/Challenge
High Treatment Costs and Limited Accessibility
- The high cost of enzyme replacement therapies and gene therapies poses a significant challenge to widespread adoption, particularly in emerging markets or regions with limited healthcare infrastructure
- For instance, olipudase alfa treatment may cost hundreds of thousands of dollars annually per patient, creating accessibility issues for many healthcare systems and patients
- The complex manufacturing and storage requirements for biologics and gene therapies further contribute to elevated prices and limited distribution
- Regulatory barriers and varying approval timelines across different countries can delay patient access to innovative therapies
- Limited awareness among healthcare providers in some regions about ASMD and its treatment options can hinder timely diagnosis and prescription of targeted therapies
- The need for continuous monitoring and frequent hospital visits for therapy administration can be burdensome for patients and caregivers, limiting adoption in certain demographics
- Reimbursement challenges and lack of insurance coverage for rare disease therapies in many countries further restrict patient access
- Logistical issues related to cold chain management and long-distance distribution of biologics can delay treatment initiation in remote or underdeveloped regions
- Potential side effects and long-term safety concerns associated with enzyme replacement therapy may also cause hesitation among physicians and patients
- Overcoming these challenges through patient assistance programs, insurance coverage, global partnerships, and technology-driven cost reduction strategies will be critical for sustained market growth
Acid Sphingomyelinase Deficiency Drugs Market Scope
The market is segmented on the basis of product and end user.
- By Product
On the basis of product, the acid sphingomyelinase deficiency drugs market is segmented into Olipudase Alfa, OKL-1014, LJPC-0712, ML-SA1, OR-0005, and Others. The Olipudase Alfa segment dominated the largest market revenue share of 46.3% in 2024, owing to its well-established therapeutic efficacy in reducing sphingomyelin accumulation and mitigating disease progression. The segment benefits from extensive clinical evidence supporting its safety and long-term outcomes, making it a preferred choice among physicians. Hospitals and specialty clinics favor Olipudase Alfa due to its predictable dosing regimen, robust patient monitoring programs, and positive impact on organ function and quality of life. The drug is widely available through regulated distribution networks, ensuring timely access for patients across multiple regions. Strategic partnerships with healthcare providers, combined with patient awareness campaigns, further strengthen its market leadership. Additionally, the segment’s dominance is reinforced by the ongoing global focus on rare disease treatment development, reimbursement support in key markets, and active participation in clinical research initiatives. Overall, Olipudase Alfa remains the backbone of the Acid Sphingomyelinase Deficiency Drugs market, maintaining a strong competitive position.
The OKL-1014 segment is expected to witness the fastest CAGR of 8.1% from 2025 to 2032, driven by its innovative therapeutic mechanism and potential to address unmet needs in enzyme replacement therapy. Its late-stage clinical trials have demonstrated promising efficacy, which is attracting attention from healthcare providers and patient advocacy groups alike. The drug’s development benefits from collaborations between biotech companies and research institutions, enhancing its clinical validation and regulatory prospects. Increased awareness of early intervention strategies, combined with patient education initiatives, is accelerating adoption in specialty clinics and hospitals. Market expansion is further supported by growing access to diagnostic facilities, improved distribution infrastructure, and favorable reimbursement policies in select regions. OKL-1014’s potential for personalized dosing and combination therapy positions it as a high-growth segment, capturing interest from both healthcare providers and patients seeking innovative treatment alternatives for Acid Sphingomyelinase Deficiency.
- By End User
On the basis of end user, the acid sphingomyelinase deficiency drugs market is segmented into hospitals, clinics, and others. The hospitals segment dominated the market with a revenue share of 58.7% in 2024, owing to their concentration of specialized medical teams, advanced treatment facilities, and comprehensive patient management capabilities. Hospitals provide structured monitoring programs for enzyme replacement therapy, ensure adherence to dosing protocols, and can manage adverse events effectively. The availability of dedicated pharmacies and well-established logistics networks enhances patient access to treatment, further cementing the segment’s leadership.
The clinics segment is expected to witness the fastest CAGR of 7.6% from 2025 to 2032, fueled by the expansion of specialty outpatient centers and the increasing preference for home-based infusion and follow-up care. Clinics are increasingly integrating patient education, adherence monitoring, and post-treatment follow-up, making them a growing contributor to overall market revenue. Rising awareness about rare disease management, alongside convenient access to specialized therapy, supports the accelerated adoption of Acid Sphingomyelinase Deficiency Drugs in this segment. The segment’s growth is further bolstered by strategic collaborations with hospitals and distribution networks, enabling clinics to provide comprehensive care in more accessible settings.
Acid Sphingomyelinase Deficiency Drugs Market Regional Analysis
- North America dominated the acid sphingomyelinase deficiency drugs market with the largest revenue share of 45.2% in 2024, driven by advanced healthcare infrastructure, high R&D investments, early adoption of novel therapies, and the strong presence of key pharmaceutical companies
- The market specifically experienced substantial growth due to active participation in clinical trials, accelerated drug approvals, and rising physician and patient awareness regarding treatment options. Early access programs, government incentives, and reimbursement policies for rare disease therapies further facilitated uptake
- The large patient population diagnosed with ASMD, combined with specialized treatment centers and established clinical networks, strengthened market growth. Collaboration between healthcare providers and pharmaceutical companies ensured wider availability of enzyme replacement therapies, while increasing awareness campaigns and support from patient advocacy groups helped educate physicians and caregivers about early diagnosis and management of ASMD
U.S. Acid Sphingomyelinase Deficiency Drugs Market Insight
The U.S. acid sphingomyelinase deficiency drugs market captured the largest revenue share within North America in 2024, fueled by a growing patient base, widespread availability of enzyme replacement therapies, and robust clinical support networks. Government-led rare disease programs and favorable regulatory frameworks accelerated market penetration. Hospitals and specialty clinics provided focused care, enabling better patient outcomes and boosting adoption rates. Physician and patient awareness programs enhanced early diagnosis and treatment uptake. Partnerships between multinational pharmaceutical companies and local distributors facilitated improved drug accessibility. Rising R&D investment in novel ASMD therapies and orphan drug development supported sustained market growth.
Europe Acid Sphingomyelinase Deficiency Drugs Market Insight
The Europe acid sphingomyelinase deficiency drugs market is projected to expand at a substantial CAGR throughout the forecast period, driven by increasing awareness of rare diseases, well-established healthcare systems, and government support programs. Countries such as Germany, the U.K., and France are witnessing steady adoption of ASMD therapies, supported by reimbursement policies covering high-cost treatments. Strong collaboration between hospitals, research institutions, and pharmaceutical companies ensures early diagnosis and therapy access. Patient advocacy and education programs in Europe contribute to better understanding and uptake of ASMD treatments. Growing investment in rare disease research and clinical trials enhances treatment innovation and availability. Expansion of specialized care centers and clinics across Europe improves patient access to enzyme replacement therapies.
U.K. Acid Sphingomyelinase Deficiency Drugs Market Insight
The U.K. acid sphingomyelinase deficiency drugs market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by government-led rare disease initiatives and expanded access to orphan drugs. Rising physician and patient awareness facilitates early diagnosis and therapy adoption. National Health Service (NHS) programs supporting rare diseases improve accessibility and affordability of ASMD treatments. Collaborations with multinational pharmaceutical companies enhance treatment availability. Patient advocacy groups contribute to improved disease understanding and treatment uptake. Specialized treatment centers ensure better clinical support and monitoring for ASMD patients.
Germany Acid Sphingomyelinase Deficiency Drugs Market Insight
The Germany acid sphingomyelinase deficiency drugs market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced diagnostic infrastructure and early adoption of innovative therapies. Favorable reimbursement policies for rare disease treatments support market growth. Hospitals, research institutions, and pharmaceutical companies collaborate to improve patient access to therapies. Rising awareness among healthcare professionals enhances early detection and management of ASMD. Investments in rare disease clinical trials promote the development of new enzyme replacement therapies. Germany’s focus on innovation and specialized care strengthens market penetration and patient outcomes.
Asia-Pacific Acid Sphingomyelinase Deficiency Drugs Market Insight
The Asia-Pacific acid sphingomyelinase deficiency drugs market is expected to grow at the fastest CAGR of 8.3% from 2025 to 2032, driven by increasing healthcare expenditure, improved diagnostic facilities, and rising awareness among physicians and patients. Countries such as China, Japan, and India are witnessing accelerated adoption due to expanding hospital networks, government-led rare disease programs, and partnerships with multinational pharmaceutical companies. Growing investments in orphan drug development enhance accessibility and affordability of ASMD therapies. Awareness campaigns by patient organizations and healthcare providers improve early diagnosis and treatment adoption. Expansion of specialty clinics and hospital networks in urban and semi-urban areas facilitates better patient reach. Regional government initiatives to strengthen rare disease management programs further contribute to market growth.
Japan Acid Sphingomyelinase Deficiency Drugs Market Insight
The Japan acid sphingomyelinase deficiency drugs market is gaining momentum due to strong focus on rare disease research and government support for orphan drug approvals. Advanced hospital infrastructure and specialized care centers enable early diagnosis and timely treatment. Growing patient and physician awareness enhances therapy adoption. Collaborative programs between government, hospitals, and pharmaceutical companies improve patient access. Aging population and increased prevalence of metabolic disorders drive demand for enzyme replacement therapies. Investment in innovative therapies and clinical trials supports sustained market expansion.
China Acid Sphingomyelinase Deficiency Drugs Market Insight
The China acid sphingomyelinase deficiency drugs market is expected to witness the highest CAGR in the Asia-Pacific region during the forecast period, driven by a growing population of diagnosed ASMD patients and expansion of rare disease programs. Government policies and initiatives promote increased healthcare access in urban and semi-urban areas. Collaborations between domestic pharmaceutical companies and global biopharmaceutical firms enhance therapy availability. Expansion of hospital networks and specialty clinics improves patient access to treatment. Rising patient and physician awareness ensures early diagnosis and adoption of therapies. Investment in research, development, and clinical trials supports the introduction of new and improved enzyme replacement therapies.
Acid Sphingomyelinase Deficiency Drugs Market Share
The acid sphingomyelinase deficiency drugs industry is primarily led by well-established companies, including:
- Catalyst Pharmaceuticals, Inc. (U.S.)
- Sanofi (France)
- Johnson & Johnson and its affiliates (U.S.)
- Orchard Therapeutics plc (U.K.)
- Lexicon Pharmaceuticals, Inc. (U.S.)
- Orphazyme (Denmark)
- Amicus Therapeutics, Inc. (U.S.)
- Swedish Orphan Biovitrum AB (Sweden)
- BridgeBio Pharma, Inc. (U.S.)
Latest Developments in Global Acid Sphingomyelinase Deficiency Drugs Market
- In March 2022, Sanofi announced that Xenpozyme (olipudase alfa) received approval in Japan under the SAKIGAKE (or “pioneer”) designation, marking the first approval for olipudase alfa anywhere in the world
- In June 2022, the European Commission approved Xenpozyme (olipudase alfa) as the first and only treatment for non-central nervous system (non-CNS) manifestations of Acid Sphingomyelinase Deficiency (ASMD) in pediatric and adult patients
- In August 2022, the U.S. Food and Drug Administration (FDA) approved Xenpozyme (olipudase alfa) for intravenous infusion in pediatric and adult patients with ASMD, making it the first approved medication to treat symptoms that are not related to the central nervous system in patients with ASMD
- In February 2022, Sanofi reported positive results from long-term, open-label extension studies demonstrating that olipudase alfa provided sustained improvement in lung function and reduction of spleen and liver volumes in adult and pediatric patients with non-CNS manifestations of ASMD
- In February 2025, a study reported the results of two years of compassionate use of olipudase alfa in an 8-month-old ASMD child, highlighting its potential in treating very young children with severe neurovisceral phenotypes of ASMD
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.