Global Acute Intermittent Porphyria Market
Market Size in USD Billion
CAGR :
% 
 USD
4.60 Billion
USD
7.28 Billion
2024
2032
USD
4.60 Billion
USD
7.28 Billion
2024
2032
| 2025 –2032 | |
| USD 4.60 Billion | |
| USD 7.28 Billion | |
|
|
|
|
Acute Intermittent Porphyria Market Size
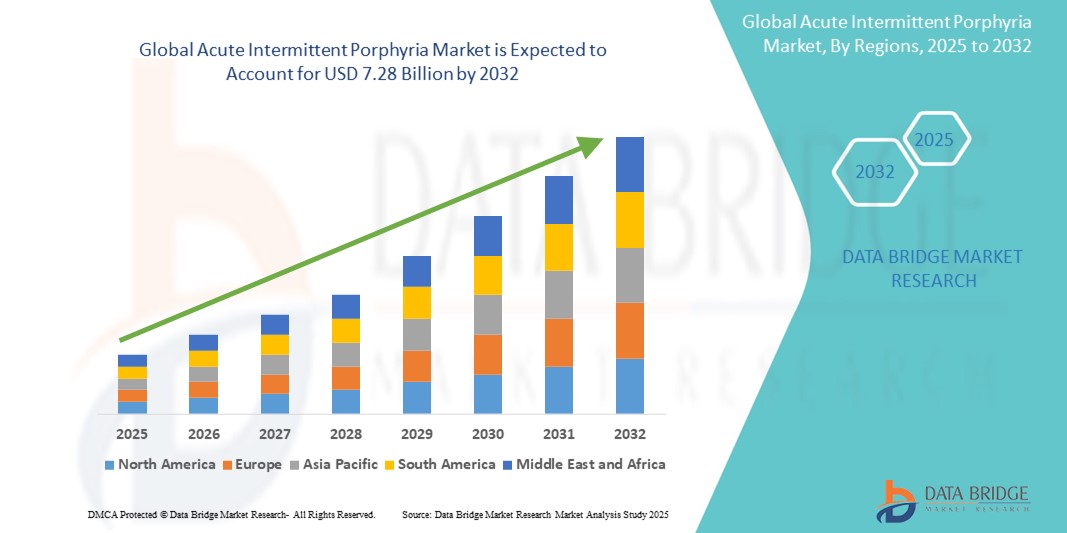
- The global acute intermittent porphyria market size was valued at USD 4.60 billion in 2024 and is expected to reach USD 7.28 billion by 2032, at a CAGR of 5.9% during the forecast period
- This growth is driven by factors such as the advancements in diagnostic technologies, increasing awareness and education, and development of novel therapies
Acute Intermittent Porphyria Market Analysis
- Acute intermittent porphyria (AIP) is a rare genetic disorder characterized by a deficiency of the enzyme porphobilinogen deaminase (PBGD), which leads to the buildup of toxic porphyrin precursors, causing severe neurological and gastrointestinal symptoms
- The demand for effective AIP treatments is significantly driven by increasing awareness about rare genetic disorders, advancements in genetic testing, and the availability of innovative therapies such as givosiran (Givlaari) and emerging gene therapies
- North America is expected to dominate the acute intermittent porphyria market with 45.6% share due to well-established healthcare infrastructure, supportive regulatory policies, and significant investments in rare disease research and development
- Asia-Pacific is expected to be the fastest-growing region in the acute intermittent porphyria market with a share of 14.2%, driven by rising awareness about rare genetic disorders, improving healthcare infrastructure, and increasing investments in precision medicine
- Urine test segment is expected to dominate the market with a market share of 53.5%, due to its non-invasive nature, cost-effectiveness, and high accuracy in detecting elevated porphyrin levels
Report Scope and Acute Intermittent Porphyria Market Segmentation
|
Attributes |
Acute Intermittent Porphyria Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Acute Intermittent Porphyria Market Trends
“Increasing Focus on Precision Medicine and The Development of Targeted Therapies”
- One prominent trend in the evolution of the acute intermittent porphyria market is the increasing focus on precision medicine and the development of targeted therapies to address the underlying genetic causes of the disease
- These advancements are transforming the management of AIP by enabling early diagnosis, reducing the frequency of acute attacks, and improving long-term patient outcomes through personalized treatment approaches
- For instance, RNA interference (RNAi) therapies such as Givlaari (givosiran) have demonstrated significant potential in reducing porphyrin precursor levels, thereby minimizing the frequency and severity of AIP attacks. In addition, emerging gene therapies targeting the root cause of the enzyme deficiency offer the possibility of a one-time curative approach
- These innovations are reshaping the AIP treatment landscape, improving the quality of life for patients, and driving the demand for advanced diagnostic tools and targeted therapies
Acute Intermittent Porphyria Market Dynamics
Driver
“Increasing Prevalence of Acute Intermittent Porphyria”
- The increasing prevalence of acute intermittent porphyria, a rare genetic disorder characterized by severe abdominal pain, neurological symptoms, and potentially life-threatening attacks, is significantly driving the demand for innovative therapies
- As awareness of rare diseases increases and diagnostic capabilities improve, more patients are being accurately diagnosed with AIP, highlighting the critical need for effective, targeted treatments
- In addition, the availability of advanced genetic testing has made it possible to identify AIP at an earlier stage, allowing for more timely interventions and improved patient outcomes
For instance,
- In June 2024, according to a report by the National Institutes of Health (NIH), the global incidence of acute porphyrias, including AIP, has been steadily rising, with an estimated prevalence of 1 in 20,000 individuals. This growing patient population is creating significant demand for novel therapies and personalized medicine approaches
- As a result, the demand for innovative treatments, such as RNA interference (RNAi) therapies and gene-editing technologies, is expected to increase as more patients seek effective management options for this debilitating condition
Opportunity
“Expanding Therapeutic Options with Gene Therapies and RNAi”
- Gene therapies and RNA interference (RNAi) are emerging as promising approaches for treating AIP by targeting the root cause of the disease, offering long-term benefits and potentially curative outcomes
- These advanced therapies work by silencing the defective genes responsible for the accumulation of toxic porphyrin precursors, thereby reducing the frequency and severity of acute attacks
- In addition, these approaches can improve the quality of life for AIP patients by reducing the need for frequent hospitalizations and emergency interventions
For instance,
- In October 2024, Alnylam Pharmaceuticals announced the successful results of a Phase 3 clinical trial for Givlaari (givosiran), an RNAi therapeutic specifically designed for AIP. The trial demonstrated a significant reduction in attack frequency and improved patient-reported outcomes, further validating the potential of RNAi-based treatments for this rare condition
- The ongoing advancements in gene-editing technologies, such as CRISPR, also present significant growth opportunities, as they aim to provide a one-time, potentially curative solution for AIP patients
Restraint/Challenge
“High Treatment Costs and Limited Access”
- The high cost of AIP therapies, particularly advanced treatments such as RNAi drugs and gene therapies, poses a significant barrier to market growth, especially in low- and middle-income regions
- These cutting-edge therapies often come with high price tags, making them less accessible to patients without comprehensive insurance coverage or government support.
- This financial barrier can limit the adoption of innovative therapies, slowing market growth and reducing the potential impact of these breakthrough treatments
For instance,
- In September 2024, a report by the Institute for Clinical and Economic Review (ICER) highlighted the high costs associated with AIP therapies, estimating that the annual treatment cost for a single AIP patient can exceed USD 400,000 in the U.S. This creates significant challenges for healthcare systems attempting to provide affordable care for rare disease patients
- As a result, the high cost of these therapies can limit their widespread adoption, particularly in regions with limited healthcare budgets and weaker reimbursement systems
Acute Intermittent Porphyria Market Scope
The market is segmented on the basis of diagnosis, treatment and end user.
|
Segmentation |
Sub-Segmentation |
|
By Diagnosis |
|
|
By Treatment |
|
|
By End User |
|
In 2025, the urine test is projected to dominate the market with a largest share in diagnosis segment
The urine test segment is expected to dominate the acute intermittent porphyria diagnosis market with the largest share of 53.5% in 2025 due to its non-invasive nature, cost-effectiveness, and high accuracy in detecting elevated porphyrin levels. As a widely used method for diagnosing AIP, its ability to provide reliable results has contributed to its market dominance. The increasing availability of advanced diagnostic technologies and growing awareness of rare diseases are expected to further drive the demand for urine tests in the diagnosis of AIP.
The gonadotropin-releasing hormone analogues is expected to account for the largest share during the forecast period in treatment market
In 2025, the gonadotropin-releasing hormone analogue segment is expected to dominate the market with the largest market share of 52.6% due to their effectiveness in reducing the frequency and severity of AIP attacks by regulating hormone levels that trigger acute symptoms. As the leading treatment option for managing AIP, advancements in therapeutic technologies and the growing focus on precision medicine are expected to further drive market growth. Increased awareness and better diagnosis of AIP will contribute to the segment's market dominance.
Acute Intermittent Porphyria Market Regional Analysis
“North America Holds the Largest Share in the Acute Intermittent Porphyria Market”
- North America dominates the acute intermittent porphyria market, holding 45.6% market share, driven by advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and a strong presence of key market players
- U.S. holds a significant share of the North American market, representing about 30.2% market share, due to the increasing demand for effective treatments for rare diseases such as acute intermittent porphyria, advancements in diagnostic and therapeutic options, and growing awareness of genetic disorders
- The availability of well-established reimbursement policies and growing investments in research & development by leading pharmaceutical and medical device companies further strengthen the market
- In addition, the increasing diagnosis of rare diseases, coupled with the growing focus on precision medicine, is fueling market expansion across the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Acute Intermittent Porphyria Market”
- Asia-Pacific is expected to witness the highest growth rate in the acute intermittent porphyria market, with an estimated 14.2% of the global market share, driven by rapid expansion in healthcare infrastructure, increasing awareness about rare diseases, and rising genetic testing volumes
- Countries such as China, India, and Japan are emerging as key markets due to the growing focus on genetic diseases, increasing healthcare access, and a rising number of healthcare initiatives aimed at tackling rare diseases
- Japan, with its advanced medical technologies and increasing healthcare investments, remains a crucial market for acute intermittent porphyria treatments and diagnostic services. The country continues to lead in the adoption of advanced diagnostic equipment and therapies for rare diseases
- India is projected to register the highest CAGR in the market, driven by expanding healthcare infrastructure, rising awareness about rare genetic disorders, and increasing demand for genetic testing and specialized treatments
Acute Intermittent Porphyria Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Recordati Rare Diseases (Italy)
- CLINUVEL PHARMACEUTICALS LTD (Australia)
- Mitsubishi Tanabe Pharma Corporation (Japan)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.