Global Adams Oliver Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
28.55 Million
USD
36.87 Million
2024
2032
USD
28.55 Million
USD
36.87 Million
2024
2032
| 2025 –2032 | |
| USD 28.55 Million | |
| USD 36.87 Million | |
|
|
|
|
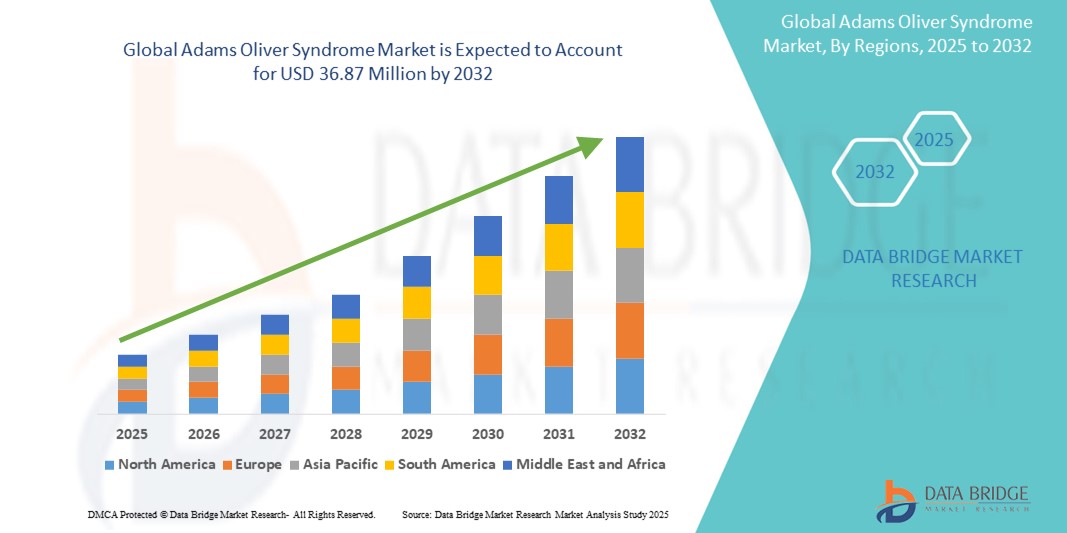
Adams Oliver Syndrome Market Size
- The Global Adams Oliver Syndrome Market size was valued at USD 28.55 billion in 2024 and is expected to reach USD 36.87 million by 2032, at a CAGR of 3.25% during the forecast period
- This growth is driven by increasing awareness of rare genetic disorders, advancements in diagnostic technologies, supportive regulatory frameworks for orphan drugs, and growing research efforts in gene therapy and targeted treatments
Adams Oliver Syndrome Market Analysis
- Adams Oliver Syndrome is a rare congenital condition characterized by abnormalities in the scalp, limbs, and vascular system. The management of AOS includes a multidisciplinary approach involving pharmacological treatment, surgical interventions, and supportive therapies
- The demand for treatments targeting AOS is driven by increasing recognition and diagnosis of the condition, ongoing clinical trials for gene-based and regenerative therapies, and the introduction of orphan drug designations by regulatory bodies such as the FDA and EMA
- North America is expected to lead the AOS market with a market share of 38.7%, owing to advanced genetic research capabilities, supportive healthcare policies for rare diseases, and the presence of key academic and biopharmaceutical institutions engaged in AOS research
- Europe is expected to be the second-largest region due to favorable reimbursement policies, awareness initiatives, and strong collaboration between research centers and healthcare providers
- The Silver Sulfadiazine segment is expected to dominate the drug market with a market share of 42.1%, attributed to its widespread use in treating wound infections and promoting healing in scalp and limb defects commonly associated with AOS. Its long-standing clinical efficacy, availability, and cost-effectiveness continue to support its leading position despite emerging treatment alternatives such as gene therapy and advanced wound care solutions
Report Scope and Adams Oliver Syndrome Market Segmentation
|
Attributes |
Adams Oliver Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Adams Oliver Syndrome Market Trends
“Rising Focus on Rare Genetic Disorder Management”
- One prominent trend in the Adams Oliver Syndrome (AOS) market is the increasing global emphasis on rare genetic disorder management
- The growing recognition of AOS and advancements in genetic research have led to the development of more targeted diagnostic tools and emerging therapies
- For instance, next-generation sequencing (NGS) and whole-exome sequencing are being adopted more widely for accurate and early diagnosis of AOS in neonates with scalp and limb defects
- This trend is transforming the clinical landscape for rare congenital disorders by promoting earlier intervention, expanding therapeutic options, and supporting the integration of personalized medicine approaches
- As rare disease registries expand and collaborations between rare disease networks grow, the AOS market is positioned to benefit from improved data collection, patient monitoring, and global awareness
- The global Adams Oliver Syndrome market is poised for growth as governments and non-profits continue to push rare disease initiatives and as emerging therapies, including gene editing and regenerative medicine, are integrated into clinical trials for congenital vascular anomalies and scalp-limb malformation
Adams Oliver Syndrome Market Dynamics
Driver
“Advancements in Genetic Testing and Precision Diagnosis”
- Rapid progress in genetic testing methods is significantly driving the AOS market
- Techniques like whole-genome sequencing, chromosomal microarray analysis, and CRISPR-based diagnostics now enable more accurate and earlier detection of Adams Oliver Syndrome, even in complex or atypical cases
- Improved diagnostic capabilities facilitate better understanding of the underlying genetic mutations (e.g., ARHGAP31, DOCK6, EOGT), allowing clinicians to tailor care pathways based on individual genotypes
- This enhances clinical outcomes and aids in genetic counseling for families affected by AOS
For instance,
- Genetic testing is now being used not just for diagnosis but also for tracking disease inheritance patterns, which is essential for early intervention and clinical decision-making.
- As precision medicine becomes more accessible globally, healthcare providers are increasingly integrating genetic testing into neonatal screening programs, further boosting demand in the AOS market
Opportunity
“High Strategic Collaborations and Orphan Drug Designation”
- The rarity of Adams Oliver Syndrome presents a major opportunity through orphan drug designation and strategic collaborations
- Regulatory agencies like the FDA and EMA offer incentives such as market exclusivity, fast-track approvals, and tax credits to encourage development of therapies for rare diseases like AOS
For instance,
- Collaborations between biotech companies and rare disease advocacy groups have led to clinical research programs focused on developing gene therapy or biologic-based treatments for syndromic disorders
- Strategic partnerships between pharmaceutical firms, academic institutions, and clinical networks support the pooling of resources and expertise for efficient drug development
- These collaborations are critical in overcoming the commercial challenges of small patient populations and accelerating innovation pipelines in rare disease treatment
- As personalized therapies and novel drug platforms continue to evolve, the AOS market stands to benefit from increased research funding and a more favorable regulatory landscape
Restraint/Challenge
“Limited Awareness and Access to Specialized Care”
- One major challenge in the AOS market is the limited awareness of the syndrome among healthcare providers and patients, especially in low- and middle-income regions
- Due to its rarity, Adams Oliver Syndrome is often misdiagnosed or not diagnosed at all, leading to delayed treatment and inadequate care
- Furthermore, specialized multidisciplinary care involving dermatologists, geneticists, neurologists, and orthopedists is often unavailable in many healthcare settings
For instance,
- In many developing countries, patients with AOS are managed only for superficial symptoms due to lack of access to genetic testing facilities or tertiary care centers
- This challenge is compounded by insufficient funding for rare disease programs and a lack of dedicated treatment protocols in general practice
- As a result, patients often face diagnostic delays, mismanagement, or financial burdens, thereby hindering early intervention and optimal outcomes
- Addressing this challenge will require focused awareness campaigns, international collaboration, and investments in rare disease infrastructure to ensure equitable care access
Adams Oliver Syndrome Market Scope
The market is segmented on the basis of drugs, treatment, mode of administration, distribution channel and end user.
|
Segmentation |
Sub-Segmentation |
|
By Drugs |
|
|
By Treatment |
|
|
By Mode of Administration |
|
|
By Distribution Channel |
|
|
By End User |
|
In 2025, Silver Sulfadiazine is projected to dominate the market with the largest share in the drugs segment
The Silver Sulfadiazine segment is expected to dominate the global Adams Oliver Syndrome market with the largest share of 42.1% in 2025, owing to its established role in managing skin ulcers, infections, and wound healing complications commonly seen in patients with Adams Oliver Syndrome. Known for its broad-spectrum antimicrobial properties, Silver Sulfadiazine is frequently used to treat congenital scalp and limb defects that are prone to secondary infections. Its widespread clinical acceptance, cost-effectiveness, and proven efficacy in wound management support its leading position among therapeutic agents. Despite the availability of other antibiotics and supportive treatments, Silver Sulfadiazine continues to be the first-line choice in many healthcare settings for managing dermatological manifestations of the syndrome.
The injectable segment is expected to account for the largest share during the forecast period in the mode of administration segment
In 2025, the injectable segment is projected to dominate the Adams Oliver Syndrome market with a market share of 57.3%, driven by its rapid onset of action, higher bioavailability, and widespread use in hospital-based treatment protocols for managing acute complications such as seizures, infections, and vascular abnormalities. In patients requiring immediate intervention, injectables provide an efficient route for delivering antibiotics, anticonvulsants, and supportive medications. This mode of administration is especially critical in neonatal and pediatric patients, where oral medication may not be feasible or effective. Additionally, the increasing hospital admissions for rare congenital disorders and the need for precision dosing in severe cases further support the dominance of injectable formulations during the forecast period
Adams Oliver Syndrome Market Regional Analysis
“North America Holds the Largest Share in the Adams Oliver Syndrome Market”
- North America dominates the Adams Oliver Syndrome market with a market share of 41.7%, owing to the presence of advanced genetic testing capabilities, increased diagnosis rates, and strong awareness regarding rare congenital disorders
- The U.S. accounts for approximately 75.9% of the North American market, driven by high healthcare spending, the presence of specialized pediatric and genetic research centers, and access to innovative treatment modalities including gene therapies and advanced wound care products
- Key pharmaceutical companies and biotech firms in the region, such as Dr. Reddy’s Laboratories and Alkem Labs, are investing in research and clinical trials targeting rare diseases, including Adams Oliver Syndrome. This facilitates the availability of a broader treatment portfolio and improved access to cutting-edge therapies
- Additionally, favorable regulatory frameworks and rare disease funding programs, such as the Orphan Drug Act in the U.S., support market growth by accelerating drug development and approvals. These factors collectively contribute to North America's dominant position in the global market
“Asia-Pacific is Projected to Register the Highest CAGR in the Adams Oliver Syndrome Market”
- The Asia-Pacific region is projected to experience the highest compound annual growth rate (CAGR) in the Adams Oliver Syndrome market during the forecast period, driven by a rising focus on rare disease diagnosis, government support, and improvements in pediatric healthcare infrastructure
- Countries such as India, China, and Japan are key growth drivers due to increasing awareness of congenital disorders, growing investments in clinical genetics, and the expansion of neonatal screening programs
- Japan continues to lead the region in terms of genetic diagnostics and neonatal care, with its strong infrastructure and adoption of personalized medicine approaches. This enhances the early identification and intervention for conditions like Adams Oliver Syndrome
- The region’s growing pharmaceutical manufacturing capacity and active participation in global clinical trials are also improving access to advanced treatment options. These developments, along with increasing healthcare expenditure, position the Asia-Pacific region as the fastest-growing market for Adams Oliver Syndrome therapies
Adams Oliver Syndrome Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Spinal Technology, Inc. (U.S.)
- Fillauer LLC (U.S.)
- LTI / Liberating Technologies, Inc. (U.S.)
- Blatchford Limited (UK)
- Ottobock (Germany)
- Hanger, Inc. (U.S.)
- Össur (Iceland)
- PROTEOR (France)
- Steeper Inc. (UK)
- Alkem Labs (India)
- Dr. Reddy’s Laboratories Ltd. (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.