Global Adenosine Deaminase Deficiency Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.71 Billion
USD
4.95 Billion
2025
2033
USD
1.71 Billion
USD
4.95 Billion
2025
2033
| 2026 –2033 | |
| USD 1.71 Billion | |
| USD 4.95 Billion | |
|
|
|
|
Adenosine Deaminase Deficiency Treatment Market Size
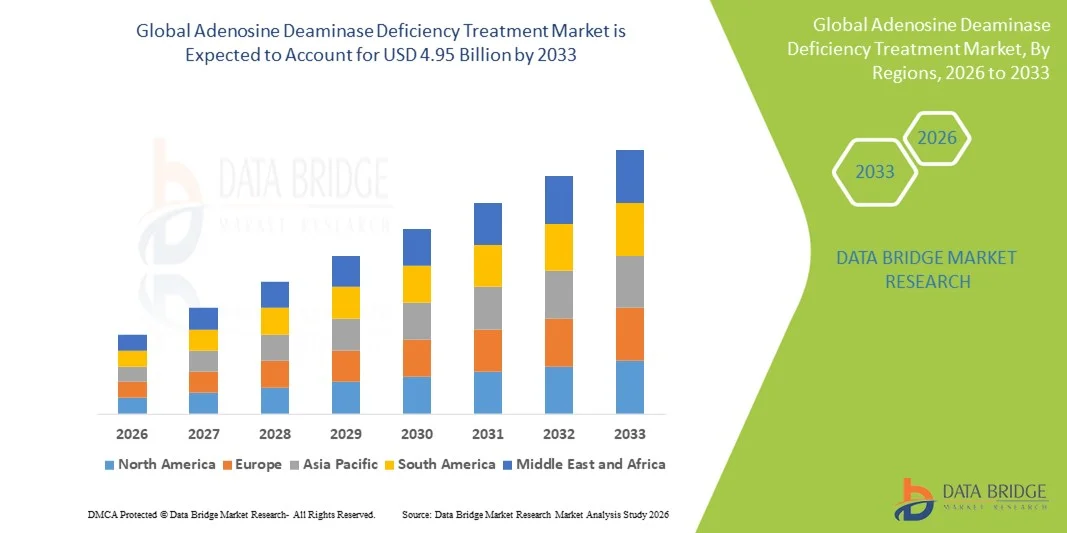
- The global adenosine deaminase deficiency treatment market size was valued at USD 1.71 billion in 2025 and is expected to reach USD 4.95 billion by 2033, at a CAGR of 14.20% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced therapies such as gene therapy, enzyme replacement therapy (ERT), and hematopoietic stem cell transplantation (HSCT), as well as expanding newborn screening programs and rising awareness of ADA-SCID
- Furthermore, regulatory support for orphan drugs, growing healthcare infrastructure in emerging markets, and rising demand for curative and effective treatments for rare immunodeficiency disorders are driving the uptake of ADA deficiency therapies, thereby significantly boosting the industry's growth
Adenosine Deaminase Deficiency Treatment Market Analysis
- Adenosine deaminase deficiency treatment, including enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), and gene therapy, is increasingly vital in managing severe combined immunodeficiency (ADA-SCID) in both pediatric and adult patients due to its ability to restore immune function and improve survival outcomes
- The escalating demand for adenosine deaminase deficiency treatment is primarily fueled by growing awareness and early diagnosis through expanded newborn screening programs, rising investment in advanced therapies, and increasing patient preference for curative and long-term treatment options
- North America dominated the adenosine deaminase deficiency treatment market with the largest revenue share of 42.9% in 2025, characterized by advanced healthcare infrastructure, early adoption of gene therapies, and a strong presence of key biopharmaceutical companies, with the U.S. experiencing substantial growth driven by regulatory support for orphan drugs and active clinical development programs
- Asia-Pacific is expected to be the fastest growing region in the adenosine deaminase deficiency treatment market during the forecast period due to improving healthcare access, rising awareness of rare immunodeficiency disorders, and increasing government initiatives to support rare disease therapies
- Gene therapy segment dominated the adenosine deaminase deficiency treatment market with a market share of 50.2% in 2025, driven by its potential curative effect, strong clinical efficacy, and a growing pipeline of next-generation therapies under development
Report Scope and Adenosine Deaminase Deficiency Treatment Market Segmentation
|
Attributes |
Adenosine Deaminase Deficiency Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Adenosine Deaminase Deficiency Treatment Market Trends
Advancements in Gene Therapy and Curative Treatments
- A significant and accelerating trend in the global adenosine deaminase deficiency treatment market is the increasing adoption of gene therapy, which offers potential curative outcomes for ADA-SCID patients and reduces the need for lifelong enzyme replacement therapy
- For instance, Strimvelis, a retroviral-based gene therapy, has demonstrated long-term immune system restoration in pediatric patients, driving interest in next-generation gene therapies across the market
- Technological progress in personalized medicine enables therapies to be tailored to individual patient genotypes, improving efficacy and minimizing adverse effects. For instance, newer lentiviral-based gene therapies under development aim to enhance safety profiles and long-term outcomes
- Integration of gene therapy with supportive care measures, such as enzyme replacement therapy during initial treatment phases, allows for smoother transitions and better patient outcomes. For instance, combining ERT with emerging gene therapies helps stabilize patients while the therapy takes effect
- This trend towards more precise, effective, and potentially curative therapies is reshaping treatment paradigms for ADA deficiency, driving research investments and adoption of innovative approaches. For instance, biopharmaceutical companies are increasingly partnering to accelerate clinical development of next-generation gene therapies
- The demand for advanced, curative adenosine deaminase deficiency treatments is growing rapidly across both pediatric and adult populations, as patients and caregivers increasingly prioritize long-term solutions over chronic enzyme therapy
Adenosine Deaminase Deficiency Treatment Market Dynamics
Driver
Rising Awareness and Early Diagnosis Programs
- The increasing prevalence of awareness programs about rare immunodeficiency disorders, coupled with expanded newborn screening initiatives, is a significant driver for the growing adoption of adenosine deaminase deficiency treatments
- For instance, in March 2025, the U.S. National Newborn Screening Program expanded ADA-SCID testing coverage, improving early detection rates and subsequent treatment uptake
- Early diagnosis allows timely intervention, which is critical for survival and long-term outcomes, motivating both physicians and caregivers to pursue advanced therapies. For instance, infants diagnosed at birth can benefit from gene therapy before severe infections occur
- Growing investments in rare disease R&D and availability of regulatory incentives for orphan drugs are further driving market growth. For instance, companies are leveraging fast-track approvals and priority review vouchers to accelerate gene therapy commercialization
- Increased patient advocacy and education initiatives are enhancing market penetration, particularly in developed regions with established healthcare infrastructures. For instance, patient support organizations are actively promoting awareness about treatment options and clinical trials
Restraint/Challenge
High Cost and Accessibility Limitation
- The high cost of advanced adenosine deaminase deficiency treatments, especially gene therapies, poses a significant challenge to broader adoption in both developed and emerging markets
- For instance, the list price of Strimvelis can exceed USD 600,000 per treatment, limiting accessibility for many patients and payers
- Limited availability of specialized treatment centers and trained healthcare professionals further restricts patient access, particularly in regions with underdeveloped healthcare infrastructure. For instance, many hospitals in emerging markets lack the facilities to administer gene therapy safely
- Regulatory and reimbursement hurdles, varying by country, can delay market entry and adoption for new therapies. For instance, differences in national healthcare policies may slow approval and coverage for gene therapies
- While financial assistance programs and expanded insurance coverage are emerging, the high upfront cost remains a barrier for widespread adoption. For instance, only a few national health systems currently cover full costs for curative gene therapy, requiring out-of-pocket payments in many cases
- Overcoming these challenges through innovative pricing models, expanded treatment access programs, and supportive policy frameworks will be vital for sustained market growth. For instance, outcome-based payment models are being explored to improve affordability and adoption
Adenosine Deaminase Deficiency Treatment Market Scope
The market is segmented on the basis of drug type, treatment, dosage, route of administration, diagnosis, end-users, and distribution channel.
- Drug Type
On the basis of drug type, the adenosine deaminase deficiency treatment market is segmented into Adagen and Revcovi. The Adagen segment dominated the market with the largest revenue share in 2025, driven by its long-standing approval history and proven efficacy in enzyme replacement therapy (ERT) for ADA-SCID patients. Adagen is widely used across hospitals and specialized clinics due to established clinical protocols and familiarity among healthcare professionals. Its safety profile and compatibility with pediatric patients make it a preferred choice for initial management of ADA deficiency. The strong market presence and availability of treatment support programs further reinforce its leadership. Additionally, Adagen’s broad adoption in North America and Europe ensures steady demand, while continuous clinical monitoring maintains patient compliance and trust in therapy outcomes.
The Revcovi segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by increasing adoption of second-generation ERT with improved dosing schedules and reduced immunogenicity. Revcovi offers enhanced pharmacokinetics and longer shelf life compared to first-generation therapies, making it convenient for both caregivers and medical providers. Its growing popularity is supported by active promotion in emerging markets and expanding insurance coverage. The drug’s efficacy in patients previously treated with Adagen and its potential use in combination with gene therapy further contributes to its adoption. Increasing awareness programs and patient advocacy efforts also accelerate Revcovi’s uptake globally.
- By Treatment
On the basis of treatment, the adenosine deaminase deficiency treatment market is segmented into medication, gene therapy, stem cell transplant, and enzyme replacement therapy. The Gene Therapy segment dominated the market in 2025 with a market share of 50.2%, driven by its potential curative effect and long-term immune system restoration for ADA-SCID patients. Gene therapy eliminates the need for lifelong enzyme therapy in many cases, improving quality of life and reducing healthcare burden over time. Its clinical success, particularly with lentiviral- and retroviral-based therapies, has encouraged regulatory approvals and investment. The segment benefits from strong patient preference and increased clinical adoption in North America and Europe. Biopharma partnerships and pipeline expansions are further supporting market dominance. Additionally, gene therapy adoption is enhanced by specialized treatment centers that provide advanced care and monitoring for ADA patients.
The Stem Cell Transplant segment is expected to witness the fastest growth from 2026 to 2033, fueled by technological advancements in allogeneic and autologous transplantation techniques. Stem cell transplants offer potential curative benefits and are increasingly preferred in regions with well-developed healthcare infrastructure. Improved safety protocols, reduced graft-versus-host disease (GvHD) risks, and better post-transplant care have increased physician and patient confidence. Its integration with supportive therapies such as ERT during the peri-transplant period further enhances outcomes. Expanding awareness among healthcare providers and patients, coupled with increasing healthcare funding in emerging markets, supports rapid adoption.
- By Dosage
On the basis of dosage, the adenosine deaminase deficiency treatment market is segmented into injection, solution, and others. The Injection segment dominated the market with the largest revenue share in 2025, driven by the standard mode of enzyme administration and its effectiveness in delivering precise therapeutic doses. Injectable therapies are preferred for their predictable pharmacokinetics and ability to rapidly correct enzyme deficiencies in patients. In addition, healthcare professionals are familiar with injection protocols, reducing administration errors and ensuring consistent patient outcomes. Accessibility in hospitals and clinics further supports widespread use. Injection therapies also allow easier integration with supportive treatments such as gene therapy and stem cell transplants. Frequent monitoring and dosage adjustments ensure continued efficacy, reinforcing the segment’s leadership.
The Solution segment is expected to witness the fastest growth from 2026 to 2033, fueled by the development of ready-to-use formulations that reduce preparation time and improve patient convenience. Solution-based therapies are gaining adoption in pediatric and home-care settings due to simplified administration. They are particularly beneficial for patients requiring frequent dosing or those with difficult venous access. Improved stability and longer shelf life of solution formulations further enhance appeal. Increasing use in emerging markets, coupled with awareness campaigns and training for caregivers, is driving rapid uptake.
- By Route of Administration
On the basis of route of administration, the adenosine deaminase deficiency treatment market is segmented into intramuscular and others. The Intramuscular segment dominated the market in 2025 due to its established clinical protocols, reliable absorption, and strong efficacy profile for enzyme replacement therapy. Intramuscular administration allows for consistent dosing, reduces systemic side effects, and is suitable for both pediatric and adult patients. Hospitals and specialized clinics prefer intramuscular delivery for monitoring treatment responses effectively. Clinical familiarity and historical usage contribute to sustained demand. In addition, intramuscular injections facilitate integration with supportive therapies such as gene therapy, improving overall patient management. Patient adherence is easier to monitor in supervised clinical settings.
The Others segment is expected to witness the fastest growth from 2026 to 2033, driven by emerging administration routes such as subcutaneous injections and novel delivery systems that improve patient comfort and convenience. These alternatives are gaining traction due to reduced pain, self-administration feasibility, and better quality of life for chronic therapy patients. Increasing research on advanced delivery methods and adoption in home-care settings accelerates growth. Awareness campaigns and physician recommendations are also boosting adoption globally.
- By Diagnosis
On the basis of diagnosis, the adenosine deaminase deficiency treatment market is segmented into genetic testing and newborn screening. The Newborn Screening segment dominated the market in 2025, driven by early detection benefits and increased survival rates for ADA-SCID patients. Early diagnosis allows timely intervention with therapies such as gene therapy or ERT, preventing life-threatening infections. Widespread implementation of screening programs in North America and Europe enhances market penetration. Government initiatives and healthcare policies supporting mandatory newborn screening further drive dominance. Clinical awareness and advocacy by patient organizations increase uptake. Integration with confirmatory genetic testing ensures accurate diagnosis and personalized treatment planning.
The Genetic Testing segment is expected to witness the fastest growth from 2026 to 2033, fueled by technological advancements in high-throughput sequencing, reduced costs, and increasing awareness of rare immunodeficiency disorders. Genetic testing enables early identification of mutations, guiding personalized therapy selection and improving outcomes. Growing adoption in emerging markets and expanding testing infrastructure supports rapid growth. Additionally, physician preference for precise molecular diagnosis enhances adoption rates. Collaborations between biotech firms and diagnostic providers are further accelerating market penetration globally.
- By End-Users
On the basis of end-users, the adenosine deaminase deficiency treatment market is segmented into clinic, hospital, and others. The Hospital segment dominated the market in 2025, driven by availability of specialized care, advanced treatment facilities, and monitoring infrastructure required for gene therapy, stem cell transplant, and ERT administration. Hospitals provide the necessary clinical expertise, supportive care, and safety measures for high-risk patients, ensuring optimal outcomes. Well-established hospitals in North America and Europe contribute to market leadership. Hospitals also facilitate post-treatment monitoring and integration with supportive therapies. Collaboration with pharmaceutical companies for clinical trials further strengthens the hospital segment.
The Clinic segment is expected to witness the fastest growth from 2026 to 2033, fueled by increasing outpatient administration of enzyme replacement therapy and home-care support services. Clinics offer convenience for follow-up treatments and routine monitoring, reducing patient travel burden. Growing telemedicine integration and support for at-home injections are enhancing adoption. Expansion of specialized immunodeficiency clinics in emerging markets is also driving rapid growth. Physician recommendations and patient preference for less intensive care settings contribute to rising clinic adoption globally.
- By Distribution Channel
On the basis of distribution channel, the adenosine deaminase deficiency treatment market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The Hospital Pharmacy segment dominated the market in 2025 due to direct access to patients receiving treatment in hospitals, controlled dispensing, and integration with inpatient and outpatient care. Hospital pharmacies ensure proper handling, storage, and administration of complex therapies such as gene therapy and ERT. Availability of trained staff and adherence to clinical protocols reinforces reliability. Strong hospital networks in North America and Europe support segment leadership. Collaboration with treatment providers for follow-up dosing also strengthens adoption.
The Online Pharmacy segment is expected to witness the fastest growth from 2026 to 2033, fueled by increasing e-commerce adoption, patient preference for home delivery, and convenience for chronic treatment administration. Online pharmacies expand access to remote or underserved regions, improving patient adherence. Integration with telemedicine and prescription management systems enhances service quality. Rising awareness of rare disease therapies through digital campaigns further drives growth. Cost-effectiveness and time-saving benefits contribute to accelerated adoption globally.
Adenosine Deaminase Deficiency Treatment Market Regional Analysis
- North America dominated the adenosine deaminase deficiency treatment market with the largest revenue share of 42.9% in 2025, characterized by advanced healthcare infrastructure, early adoption of gene therapies, and a strong presence of key biopharmaceutical companies, with the U.S. experiencing substantial growth driven by regulatory support for orphan drugs and active clinical development programs
- Patients and caregivers in the region highly value the availability of specialized treatment centers, clinical expertise, and comprehensive care programs, which ensure timely diagnosis, effective treatment, and continuous monitoring for ADA-SCID patients
- This widespread adoption is further supported by strong regulatory support for orphan drugs, high healthcare spending, and growing awareness among physicians and patient advocacy groups, establishing adenosine deaminase deficiency treatments as a preferred solution for managing rare immunodeficiency disorders in both pediatric and adult populations
U.S. Adenosine Deaminase Deficiency Treatment Market Insight
The U.S. adenosine deaminase deficiency treatment market captured the largest revenue share of 82% in 2025 within North America, fueled by early adoption of gene therapy, expanded newborn screening programs, and advanced healthcare infrastructure. Patients and caregivers increasingly prioritize curative treatments and long-term management of ADA-SCID, driving adoption of enzyme replacement therapy and gene therapies. The growing awareness among physicians, coupled with robust insurance coverage and patient support programs, further propels the market. Moreover, ongoing clinical trials and strong presence of leading biopharmaceutical companies contribute to sustained growth. Integration of innovative therapies with supportive care solutions enhances patient outcomes and drives market expansion.
Europe Adenosine Deaminase Deficiency Treatment Market Insight
The Europe adenosine deaminase deficiency treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by well-established healthcare systems, regulatory support for rare disease therapies, and increasing awareness of ADA-SCID. The adoption of enzyme replacement therapy and gene therapy is rising across hospitals and specialty clinics. European patients and healthcare providers value early diagnosis and effective treatment options that improve survival and quality of life. The region is witnessing significant growth in pediatric and adult populations, with treatments integrated into both hospital-based programs and outpatient care. Ongoing research initiatives and collaborations between biotech companies and healthcare institutions are further accelerating market adoption.
U.K. Adenosine Deaminase Deficiency Treatment Market Insight
The U.K. adenosine deaminase deficiency treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by expanding newborn screening programs and increasing patient awareness. Concerns about long-term management of ADA-SCID are encouraging caregivers and physicians to adopt advanced therapies such as gene therapy and enzyme replacement therapy. The U.K.’s robust healthcare infrastructure, combined with widespread diagnostic availability and strong insurance support, is expected to continue stimulating market growth. Integration of treatment protocols with specialized clinics and patient support programs enhances accessibility and adherence.
Germany Adenosine Deaminase Deficiency Treatment Market Insight
The Germany adenosine deaminase deficiency treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by strong regulatory frameworks, focus on rare disease management, and increasing awareness of innovative therapies. Germany’s advanced healthcare system, clinical expertise, and emphasis on research and development promote adoption of gene therapy and stem cell transplants. Hospitals and specialty centers are increasingly integrating ADA treatments into comprehensive care plans. The market is also driven by the preference for long-term, curative treatment approaches that improve patient quality of life. Continued collaboration between biotech companies and healthcare providers supports sustained market growth.
Asia-Pacific Adenosine Deaminase Deficiency Treatment Market Insight
The Asia-Pacific adenosine deaminase deficiency treatment market is poised to grow at the fastest CAGR of 25% from 2026 to 2033, driven by improving healthcare infrastructure, rising awareness of rare immunodeficiency disorders, and increasing availability of advanced therapies. Countries such as China, Japan, and India are witnessing growth in gene therapy adoption, enzyme replacement therapy, and supportive care programs. Government initiatives promoting rare disease diagnosis and treatment accessibility are driving market expansion. Additionally, growing investments by biotech firms to enter emerging markets enhance the availability and affordability of treatments. The region’s increasing focus on specialized clinics and newborn screening programs further supports rapid adoption.
Japan Adenosine Deaminase Deficiency Treatment Market Insight
The Japan adenosine deaminase deficiency treatment market is gaining momentum due to the country’s strong healthcare infrastructure, high patient awareness, and early adoption of gene therapy. The Japanese market places emphasis on improving pediatric and adult patient outcomes, with hospitals and specialty clinics providing advanced care and follow-up. Integration of enzyme replacement therapy, gene therapy, and supportive care measures is fueling growth. Additionally, Japan’s focus on precision medicine and advanced diagnostics supports the uptake of personalized ADA treatment protocols. Aging population and the desire for efficient and safe treatment solutions in both residential and hospital settings contribute to market expansion.
India Adenosine Deaminase Deficiency Treatment Market Insight
The India adenosine deaminase deficiency treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rising awareness of rare diseases, improving healthcare access, and expanding neonatal screening programs. India is witnessing increased adoption of enzyme replacement therapy and emerging gene therapies across hospitals and specialty clinics. Government initiatives supporting rare disease treatment and affordable therapy options are key factors propelling the market. Additionally, increasing partnerships between global biopharmaceutical companies and local healthcare providers enhance accessibility. Growing diagnostic capabilities and patient support programs are further driving market penetration across pediatric and adult populations.
Adenosine Deaminase Deficiency Treatment Market Share
The Adenosine Deaminase Deficiency Treatment industry is primarily led by well-established companies, including:
- Leadiant Biosciences, Inc. (U.S.)
- BioMarin (U.S.)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- AbbVie Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- TEIJIN LIMITED (Japan)
- Actelion Pharmaceuticals US, Inc. (U.S.)
- AstraZeneca (U.K.)
- Stoke Therapeutics (U.S.)
- Spark Therapeutics, Inc. (U.S.)
- Shire PLC (U.K.)
- ADARx Pharmaceuticals (U.S.)
- Shape Therapeutics, Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- Alcediag (France)
- ProQR Therapeutics (Netherlands)
- Zoetis Services LLC (U.S.)
- Broad Institute (U.S.)
What are the Recent Developments in Global Adenosine Deaminase Deficiency Treatment Market?
- In October 2025, a long-term follow-up study of 62 children treated with lentiviral gene therapy (using autologous CD34+ cells) reported 100% overall survival and 95% event-free survival after a median of 7.5 years, with stable gene marking and no leukoproliferative complications. These results strongly support the durability and safety of this curative approach
- In May 2024, Great Ormond Street Hospital (GOSH) announced plans to license its own ADA‑SCID gene therapy on a non-profit basis after the original commercial developer (Orchard) dropped out. This is significant because it could improve affordability and accessibility for patients, making a previously costly therapy more widely available
- In April 2024, a case of T‑cell acute lymphoblastic leukemia (T‑ALL) was reported in a patient around 4.7 years after receiving γ‑retroviral (γ‑RV) gene therapy for ADA‑SCID, linked to vector insertion activating the LMO2 oncogene. This raised important long-term safety concerns about insertional mutagenesis with older retroviral vector approaches
- In July 2023, the European Commission approved the transfer of marketing authorization for the ADA‑SCID gene therapy Strimvelis from Orchard Therapeutics to the non-profit Fondazione Telethon. This move marked a shift toward non‑profit stewardship of this ultra‑rare disease therapy, potentially increasing patient access and reducing commercial pressures
- In May 2021, researchers at UCLA and Great Ormond Street Hospital (GOSH) announced that an experimental lentiviral gene therapy restored immune function in 48 of 50 children with ADA‑SCID, and the effect persisted for 2–3 years without the need for additional treatment. This was a landmark because it suggested a one-time, potentially curative treatment, unsuch as lifelong enzyme replacement therapy
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.