Global Advanced Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
11.52 Billion
USD
21.90 Billion
2024
2032
USD
11.52 Billion
USD
21.90 Billion
2024
2032
| 2025 –2032 | |
| USD 11.52 Billion | |
| USD 21.90 Billion | |
|
|
|
|
Advanced Therapeutics Market Analysis
The global advanced therapeutics market is rapidly evolving, fueled by technological advancements and the increasing prevalence of chronic and genetic diseases. The demand for cutting-edge therapies such as gene therapy, CAR-T cell therapy, and RNA-based treatments is growing, particularly in oncology, genetic disorders, and neurological diseases. For instance, the global incidence of cancer is expected to reach 28.4 million cases by 2040, as per the World Health Organization (WHO), which underscores the critical need for novel treatments. Additionally, rare diseases, affecting millions worldwide with over 7,000 recognized conditions, continue to drive interest in advanced therapeutics, as these conditions often lack effective treatment options. The increasing focus on personalized medicine, where treatments are tailored to individual genetic profiles, is also contributing to market expansion, with significant investment in research and clinical trials aimed at developing more effective and targeted therapies for diverse patient populations.
Advanced Therapeutics Market Size
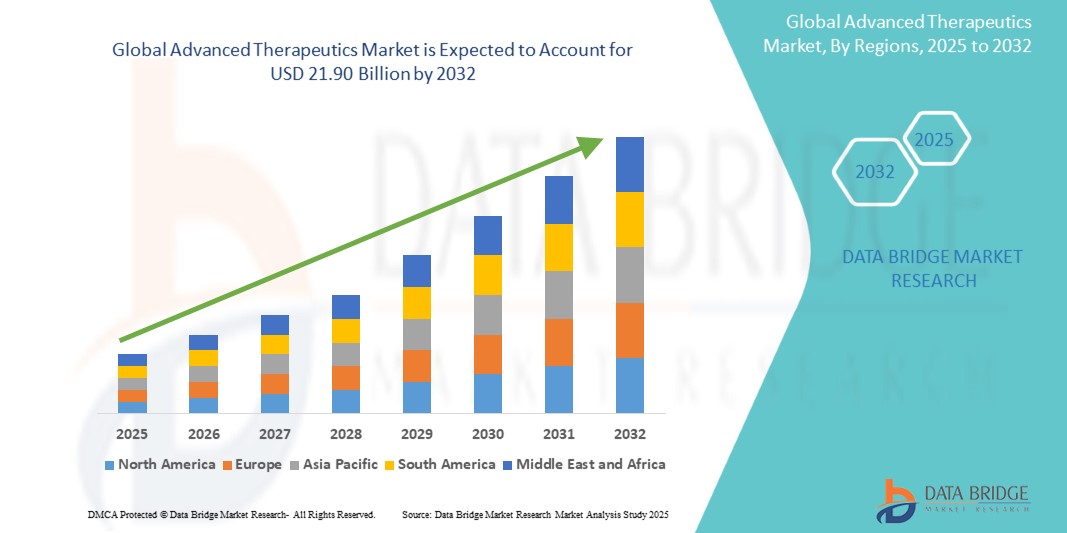
Global Advanced Therapeutics market size was valued at USD 11.52 billion in 2024 and is projected to reach USD 21.90 billion by 2032, with a CAGR of 8.40% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Advanced Therapeutics Market Trends
“Convergence of Digital Health and Therapeutics”
The convergence of digital health and therapeutics is transforming patient care. Tools such as AI, machine learning, and data analytics enhance patient monitoring, enabling real-time adjustments to treatments based on individual responses. These technologies also allow for treatment customization and more accurate outcomes prediction, helping clinicians tailor therapies to each patient. In clinical trials, digital tools improve efficiency by streamlining data collection and patient recruitment, making trials more patient-centric and accessible. This trend is advancing personalized healthcare, with a stronger focus on data-driven, tailored treatments.
Report Scope and Advanced Therapeutics Market Segmentation
|
Attributes |
Advanced Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Novartis International AG (Switzerland), Gilead Sciences, Inc. (U.S.), Amgen Inc. (U.S.), Bluebird Bio, Inc. (U.S.), Spark Therapeutics, Inc. (U.S.), Regeneron Pharmaceuticals, Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), Biogen Inc. (U.S.), AbbVie Inc. (U.S.), Sarepta Therapeutics, Inc. (U.S.), Autolus Therapeutics plc (U.K.), Cellectis S.A. (France), Intellia Therapeutics, Inc. (U.S.), Editas Medicine, Inc. (U.S.), Orchard Therapeutics (U.K.), Rocket Pharmaceuticals, Inc. (U.S.). |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Advanced Therapeutics Market Definition
Advanced therapeutics refers to a class of innovative medical treatments that leverage cutting-edge technologies, such as gene therapy, cell therapy, RNA-based therapies, and immunotherapies, to treat complex, chronic, or genetic diseases. These therapies aim to address the root causes of diseases at the molecular or cellular level, offering targeted, personalized, and more effective treatment options. Unlike traditional treatments, advanced therapeutics focus on repairing, modifying, or replacing faulty genes or cells to restore normal function, improve patient outcomes, and potentially provide long-term or permanent solutions for conditions that were previously difficult to treat.
Advanced Therapeutics Market Dynamics
Drivers
- Increasing Prevalence of Chronic and Genetic Diseases
The increasing prevalence of chronic and genetic diseases is a major driver of the global advanced therapeutics market. Conditions such as cancer, cardiovascular diseases, and genetic disorders are becoming more common due to factors such as aging populations, lifestyle changes, and genetic predispositions. These diseases often require innovative treatments that go beyond traditional therapies. For instance, gene therapies can correct or replace faulty genes, while immunotherapies such as CAR-T cell therapies are designed to enhance the body's immune system to target and destroy cancer cells. Unlike conventional treatments, which may only manage symptoms, advanced therapeutics aim to address the root causes of diseases at the molecular or cellular level. This makes them more effective in providing long-term or even permanent solutions. As the need for more targeted and personalized treatments grows, the demand for advanced therapeutics to manage and treat these chronic and genetic diseases continues to rise. For instance, in March 2024, according to an article published by nature, epidemiological studies suggest that 2–6% of the global population is affected by rare diseases, with up to 80% of these being genetic in origin. This growing prevalence of rare genetic conditions is expected to drive demand for advanced therapeutics, as novel treatments and therapies are developed to address these unmet medical needs.
- Advancements in Biotechnology and Genetic Research
Advancements in biotechnology and genetic research are significantly driving the global advanced therapeutics market. Technologies such as CRISPR gene editing are enabling precise modifications of the genome, offering potential cures for previously untreatable genetic disorders. Cell-based therapies, such as stem cell treatments, are advancing regenerative medicine, allowing for the repair or replacement of damaged tissues. Additionally, RNA-based therapies, including messenger RNA (mRNA) treatments, are emerging as powerful tools for combating infectious diseases and genetic conditions by directly targeting the genetic code. These innovations are expanding the range of diseases that can be treated, from rare genetic disorders to complex cancers. As research in biotechnology progresses, personalized and targeted treatments are becoming more feasible, allowing therapies to be tailored to individual genetic profiles and disease mechanisms. This shift toward more effective and precise therapies is transforming the landscape of healthcare, offering new hope for patients with challenging conditions. In February 2024, according to an article published by Mewburn Ellis, the FDA approved Amtagvi (lifileucel), the first cell therapy for solid tumors, developed by Iovance Biotherapeutics. It is approved for treating unresectable melanoma in patients who have not responded to PD-1 and BRAF inhibitors. This approval marks a significant milestone and is expected to drive growth in the global advanced therapeutics market by expanding treatment options for solid tumors and paving the way for future innovations in cell therapies.
Opportunities
- Increasing Partnerships and Collaborations
Partnerships and collaborations in research and development are crucial opportunities for the global advanced therapeutics market. By teaming up with biotech companies, pharmaceutical giants, and research institutions, stakeholders can share valuable resources, expertise, and technologies, significantly speeding up the development process. Collaborative efforts allow for faster clinical trials through shared data, joint patient recruitment, and pooled clinical expertise, ultimately reducing time to market for new treatments. These partnerships also enable more efficient regulatory approval processes, as combined efforts can navigate complex regulations more effectively and ensure faster access to the market. In addition to improving the speed and efficiency of development, such collaborations help tackle the challenge of high research and development costs, spreading the financial burden and enabling investment in innovative therapies. This dynamic also encourages innovation in both established and emerging markets, making advanced therapeutics more accessible to a broader patient population globally. For instance, in November 2024, Ascend Advanced Therapies has partnered with EW Healthcare Partners to expand its U.S. footprint and enhance its infrastructure. This collaboration is expected to serve as an opportunity for Ascend by enabling the company to tap into the growing U.S. market, strengthening its position in gene-to-GMP development, and accelerating investments in advanced therapeutic technologies.
- Growth of Personalized Medicine
The growth of personalized medicine represents a significant opportunity in the Global Advanced Therapeutics Market. With advancements in genetic profiling and biomarker discovery, treatments are becoming more tailored to individual patients. By analyzing a patient’s unique genetic makeup, doctors can select or design therapies that are more effective and specifically suited to their condition, ensuring higher success rates. This targeted approach not only increases the efficacy of treatments but also reduces the likelihood of adverse side effects, as therapies are designed to work with the patient’s biology. Personalized medicine is especially valuable in complex diseases such as cancer, where genetic mutations can vary widely between patients. As a result, the shift towards more customized healthcare leads to improved treatment outcomes and higher patient satisfaction, as patients receive therapies that directly address their specific needs. This trend is accelerating the growth of personalized therapeutics, making it a key area for future innovation and market expansion.
Restraints/Challenges
- High Development and Manufacturing Costs
High development and manufacturing costs are a significant restraint in the global advanced therapeutics market. Advanced therapies, such as gene therapy, cell therapy, and RNA-based treatments, require cutting-edge technologies and specialized expertise, driving up costs. The development of these therapies involves complex processes and requires state-of-the-art equipment, facilities, and skilled personnel, leading to substantial research and production expenses. Clinical trials for such therapies are also expensive, as they often require larger sample sizes, longer durations, and complex regulatory processes. These high costs translate into elevated prices for patients, making these therapies inaccessible to many healthcare systems, especially in low- and middle-income regions. Additionally, the high financial burden can create challenges in obtaining insurance reimbursement and regulatory approval, limiting market expansion. As a result, the cost barriers associated with advanced therapeutics can hinder their widespread adoption and slow the growth of the overall market. In February 2024, according to an article published by Charles River Laboratories, in 2023, the FDA approved seven new cell and gene therapies for diseases such as muscular dystrophy, sickle cell anemia, and Type 1 diabetes. While these therapies offer new hope, their high costs, ranging from USD 338,000 to USD 3.2 million, and long wait times of over two years pose significant challenges. This cost and accessibility issue may act as a restraint on the global advanced therapeutics market, limiting their widespread adoption despite their potential benefits.
- Stringent Regulatory Standards
Regulatory challenges are a significant hurdle in the global advanced therapeutics market. Advanced therapies, including gene therapies, cell therapies, and RNA-based treatments, face stringent approval processes due to their novel nature and lack of established guidelines. Regulatory bodies such as the FDA and EMA require comprehensive clinical trial data to verify safety and efficacy, often resulting in extended timelines for approval. The absence of standardized regulations for these cutting-edge treatments further complicates the approval process, leading to uncertainty for developers. Additionally, post-market monitoring and long-term safety assessments pose ongoing challenges, as the long-term effects of some therapies are not yet fully understood. This uncertainty increases costs for manufacturers, who must invest in additional studies to meet regulatory requirements. As a result, these regulatory hurdles slow innovation, delay time-to-market, and restrict the availability of advanced therapeutics, creating barriers for both manufacturers and patients seeking access to these novel treatments. In December 2020, according to an article published by NCBI, the new guidelines for ATMPs address manufacturing challenges, short shelf-lives, and issues with placebo controls. They also emphasize the need for long-term follow-up of subjects due to potential long-term effects, and acknowledge that non-clinical data may not always be available before human trials. These regulatory complexities and the need for extensive follow-up may act as a challenge for the Global Advanced Therapeutics Market, slowing down the development and approval of new therapies.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Advanced Therapeutics Market Scope
The market is segmented on the basis of technology, application, patient demographics, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Technology
- Gene Therapy
- Cell Therapy
- Tissue Engineering
- RNA-based Therapies
- Others
Application
- Cancer
- Genetic Disorders
- Cardiovascular Diseases
- Neurological Disorders
- Autoimmune Disorders
- Others
Patient Demographics
- Pediatric
- Adult
- Elderly
End-User
- Hospitals
- Research and Academic Institutions
- Biopharmaceutical Companies
- Others
Advanced Therapeutics Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, technology, application, patient demographics, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its strong healthcare infrastructure, high investment in biotechnology and pharmaceutical research, and favorable regulatory environment, which supports the development and commercialization of innovative therapies.
Asia-Pacific is expected to be the fastest growing due to several key factors. The region is witnessing a surge in healthcare investments, driven by both government initiatives and private sector funding aimed at improving healthcare infrastructure and services. The expanding biotechnology sector, supported by robust R&D activities, innovative start-ups, and collaborations with global players, is further fueling this growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Advanced Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Advanced Therapeutics Market Leaders Operating in the Market Are:
- Novartis International AG (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- Amgen Inc. (U.S.)
- Bluebird Bio, Inc. (U.S.)
- Spark Therapeutics, Inc. (U.S.)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Biogen Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Autolus Therapeutics plc (U.K.)
- Cellectis S.A. (France)
- Intellia Therapeutics, Inc. (U.S.)
- Editas Medicine, Inc. (U.S.)
- Orchard Therapeutics (U.K.)
- Rocket Pharmaceuticals, Inc. (U.S.)
Latest Developments in Advanced Therapeutics Market
- In December 2024, Dewpoint Therapeutics Inc. has partnered with Mitsubishi Tanabe Pharma Corporation (MTPC) to advance its novel TDP-43 small molecule condensate modulator for amyotrophic lateral sclerosis (ALS), effective December 1, 2024. This collaboration will help Dewpoint Therapeutics by enhancing its research capabilities, accelerating the development of its ALS treatment, and expanding its presence in the neurological therapeutics market
- In November 2024, Ascend Advanced Therapies has teamed up with EW Healthcare Partners to strengthen its presence and expand its infrastructure in the U.S. This partnership will help Ascend by enhancing its market reach, boosting operational capabilities, and supporting continued growth in gene-to-GMP development
- In November 2024, Bharath Advanced Therapeutics (BAT) and the Federation of Asian Biotech Associations (FABA) are hosting Cancer NEXT 2024, an oncology research conference aimed at advancing cancer treatment through collaboration and innovation. This event will help BAT by enhancing its visibility in the oncology sector, fostering key partnerships, and showcasing its leadership in cancer research
- In November 2024, NextCell Pharma AB, the parent company of QVance, supports QVance’s partnership with Royale International to enhance the supply chain for cell and gene therapies across Europe by leveraging Royale International’s logistics expertise. This collaboration will help QVance by improving its distribution capabilities, ensuring efficient delivery, and expanding its reach in the European cell and gene therapy market
- In October 2024, Dyno Therapeutics, Inc. has announced its second research collaboration with Roche to develop advanced AAV vectors for gene therapies targeting neurological diseases. This partnership will help Dyno Therapeutics by enhancing its AI-driven gene delivery technologies and accelerating the development of innovative therapies for neurological conditions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.