Global Ai Powered Clinical Research Platform Market
Market Size in USD Billion
CAGR :
% 
 USD
2.47 Billion
USD
9.13 Billion
2024
2032
USD
2.47 Billion
USD
9.13 Billion
2024
2032
| 2025 –2032 | |
| USD 2.47 Billion | |
| USD 9.13 Billion | |
|
|
|
|
AI-Powered Clinical Research Platform Market Size
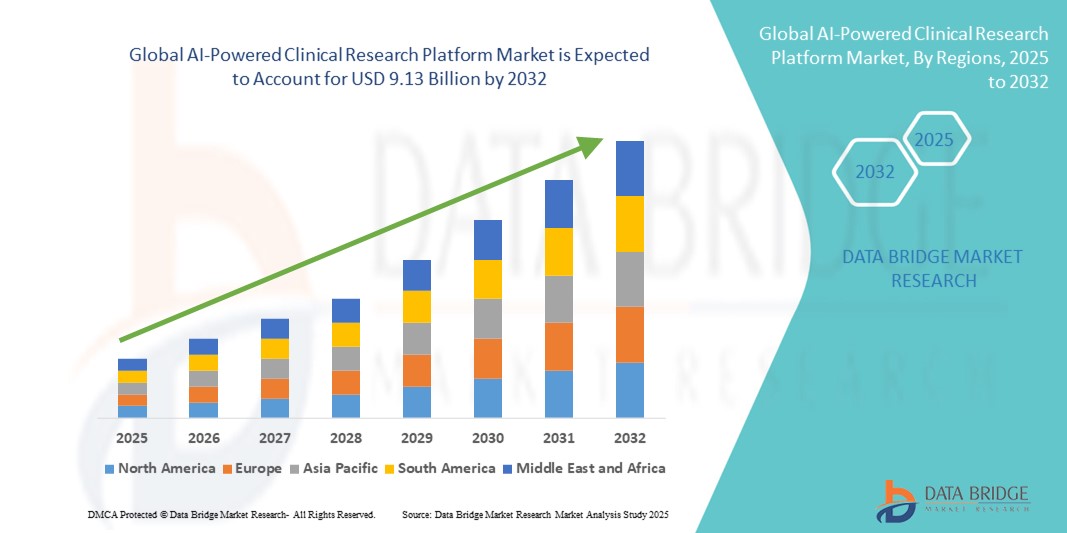
- The global AI-powered clinical research platform market size was valued at USD 2.47 billion in 2024 and is expected to reach USD 9.13 billion by 2032, at a CAGR of 17.73% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within artificial intelligence (AI) and machine learning across clinical trials, leading to increased digitalization, automation, and predictive capabilities in both early-phase and late-phase research
- Furthermore, rising demand for faster, cost-efficient, and more accurate trial outcomes is establishing AI-Powered Clinical Research Platforms as the modern standard for clinical data management and decision-making. These converging factors are accelerating the uptake of AI-Powered Clinical Research Platform solutions, thereby significantly boosting the industry's growth
AI-Powered Clinical Research Platform Market Analysis
- AI-powered clinical research platforms, offering advanced analytics, automated data processing, and intelligent patient matching, are becoming increasingly vital components of modern drug development and clinical trials due to their ability to improve trial efficiency, reduce costs, and accelerate time-to-market for new therapies
- The escalating demand for AI in clinical research is primarily fueled by the growing complexity of clinical trials, the need for real-time data analysis, and increasing regulatory pressures for data integrity and patient safety
- North America dominated the AI-powered clinical research platform market with the largest revenue share of 42.6% in 2024, characterized by early adoption of AI technologies in clinical workflows, strong presence of pharmaceutical giants, and robust investments in digital health infrastructure. The U.S. continues to lead regional growth due to innovation hubs, AI-focused startups, and favorable government initiatives supporting AI in healthcare
- Asia-Pacific is expected to be the fastest growing region in the AI-powered clinical research platform market during the forecast period, projected to grow at a CAGR of 19.3% from 2025 to 2032, driven by expanding clinical trial activities, increasing digitization in healthcare, and rising government support for AI integration in countries such as China, India, and Japan
- The cloud-based segment dominated the AI-powered clinical research platform market with a revenue share of 58.3% in 2024, supported by its scalability, lower infrastructure costs, and ease of real-time data access
Report Scope and AI-Powered Clinical Research Platform Market Segmentation
|
Attributes |
AI-Powered Clinical Research Platform Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
AI-Powered Clinical Research Platform Market Trends
Advanced Data Management and Automation in Clinical Trials
- A significant and accelerating trend in the global AI-powered clinical research platform market is the use of advanced AI algorithms for real-time data processing, patient stratification, and predictive analytics. These capabilities are enhancing trial precision, reducing timeframes, and lowering operational costs across all phases of clinical research
- For instance, leading platforms such as Deep 6 AI and Saama Technologies are helping pharmaceutical companies and contract research organizations (CROs) rapidly identify suitable patient cohorts and streamline data collection from electronic health records (EHRs), wearables, and remote monitoring tools
- The integration of machine learning and natural language processing (NLP) enables platforms to extract actionable insights from vast volumes of unstructured data such as physician notes, pathology reports, and historical clinical trial datasets
- Furthermore, automation in protocol design, site selection, and regulatory documentation is reducing administrative burdens and human error, making the trial lifecycle significantly more efficient. This automation is especially valuable in adaptive trial designs and decentralized clinical trials (DCTs), where flexibility and speed are paramount
- Cloud-based deployment models and centralized data dashboards are facilitating remote monitoring, cross-site collaboration, and real-time performance tracking, which have become vital tools in post-pandemic clinical operations
- As data security and regulatory compliance remain central concerns, AI-powered platforms are increasingly integrating blockchain and secure audit trails to ensure transparency, traceability, and integrity across all trial data points
AI-Powered Clinical Research Platform Market Dynamics
Driver
Growing Need Due to Rising Complexity of Clinical Trials and Demand for Accelerated Drug Development
- The increasing complexity of clinical trials, coupled with growing pressure to reduce time-to-market for new drugs and therapies, is a major driver for the adoption of AI-powered clinical research platforms
- For instance, in April 2024, IQVIA announced enhancements to its AI-based clinical trial optimization suite, enabling real-time patient stratification and automated protocol adjustments, which significantly reduce trial delays and drop-out rates
- With global clinical trial activity expanding and personalized medicine gaining ground, AI platforms offer researchers the ability to mine vast datasets, identify biomarkers, and match patients to trials with higher accuracy
- In addition, AI is being widely integrated into electronic data capture (EDC), site monitoring, and trial supply management, all of which are helping sponsors improve operational efficiency and compliance
- The growing focus on decentralized and hybrid trials—particularly post-pandemic—has created a need for intelligent platforms that can manage remote data capture, wearable integration, and real-time risk monitoring
Restraint/Challenge
Concerns Regarding Data Privacy, Regulatory Barriers, and Integration Costs
- Despite their promise, AI-powered clinical research platforms face challenges related to data privacy, ethical compliance, and interoperability with legacy systems
- For example, variations in data protection regulations such as GDPR in Europe and HIPAA in the U.S. can complicate the use of patient data across borders and restrict full-scale implementation of AI models
- Skepticism from regulatory authorities regarding the interpretability and transparency of AI decision-making poses a hurdle for validation and approvals in high-stakes trials, particularly in oncology and rare diseases
- Another major barrier is the high initial investment required for deployment and staff training, especially for smaller CROs and research institutions operating on tight budgets
- Further, integrating AI platforms with traditional EHRs, clinical trial management systems (CTMS), and lab data remains technically challenging, often requiring custom development or third-party middleware
- Overcoming these limitations will require collaborative standard-setting across regulators, technology providers, and research sponsors, along with the development of cost-effective, modular AI solutions tailored to different trial phases
AI-Powered Clinical Research Platform Market Scope
The market is segmented on the basis of type, technology, phase, deployment mode, application and end user
- By Type
On the basis of type, the AI-powered clinical research platform market is segmented into electronic data capture (EDC), clinical trial management systems (CTMS), eCOA/ePRO platforms, safety solutions, risk-based monitoring platforms, and others. The Electronic Data Capture (EDC) segment dominated the largest market revenue share of 38.5% in 2024, driven by the growing emphasis on streamlined data collection and compliance with regulatory standards.
The risk-based monitoring platforms segment is expected to witness the fastest growth rate of 20.4% from 2025 to 2032, due to rising demand for predictive analytics and early risk identification in clinical trials.
- By Technology
On the basis of technology, the AI-powered clinical research platform market is segmented into machine learning, natural language processing (NLP), deep learning, computer vision, and others. The Machine Learning segment held the largest market share of 42.7% in 2024, attributed to its wide-ranging applications in patient recruitment, protocol design, and data modeling.
The natural language processing (NLP) segment is anticipated to grow at the fastest CAGR of 22.8% from 2025 to 2032, owing to its ability to extract insights from unstructured data such as clinical notes and scientific publications.
- By Phase
On the basis of phase, the AI-powered clinical research platform market is segmented into Phase I, Phase II, Phase III, Phase IV, and Preclinical. The Phase III segment accounted for the largest revenue share of 34.7% in 2024, due to the high complexity and cost of late-stage trials where AI enhances efficiency and success rates.
The Preclinical segment is projected to grow at the highest CAGR of 19.1% from 2025 to 2032, driven by AI’s increasing role in early-stage drug discovery and predictive modeling.
- By Deployment Mode
On the basis of deployment mode, the AI-powered clinical research platform market is segmented into cloud-based, on-premise, and hybrid. The cloud-based segment led the market with a revenue share of 58.3% in 2024, supported by its scalability, lower infrastructure costs, and ease of real-time data access.
The hybrid segment is expected to record the fastest CAGR of 18.6% from 2025 to 2032, as organizations seek a balance between data control and cloud flexibility.
- By Application
On the basis of application, the AI-powered clinical research platform market is segmented into oncology, neurology, cardiology, infectious diseases, immunology, and others. The oncology segment dominated the market with a revenue share of 41.2% in 2024, fueled by the complexity and volume of cancer trials that benefit from AI-powered analytics.
The neurology segment is expected to grow at the fastest CAGR of 23.5% from 2025 to 2032, due to rising research into Alzheimer’s, Parkinson’s, and related disorders where AI can accelerate diagnosis and treatment discovery.
- By End User
On the basis of end user, the AI-powered clinical research platform market is segmented into pharmaceutical & biotechnology companies, contract research organizations (cros), academic research institutes, hospitals & clinics, and others. The Pharmaceutical & Biotechnology Companies segment captured the largest revenue share of 46.3% in 2024, driven by growing AI investments to enhance clinical trial productivity and R&D pipelines.
The Contract Research Organizations (CROs) segment is projected to witness the fastest CAGR of 21.9% from 2025 to 2032, as CROs increasingly adopt AI tools to offer cost-effective and efficient services to sponsors globally.
AI-Powered Clinical Research Platform Market Regional Analysis
- North America dominated the AI-powered clinical research platform market with the largest revenue share of 42.6% in 2024, driven by the strong presence of leading pharmaceutical companies, contract research organizations (CROs), and AI technology firms. The region's leadership is supported by favorable regulatory frameworks for clinical trials and significant investments in precision medicine and data-driven drug development
- Organizations in North America highly value AI-powered platforms for their ability to accelerate trial design, patient recruitment, and protocol optimization. The integration of predictive analytics, real-world evidence (RWE), and natural language processing (NLP) further strengthens platform capabilities across various trial phases
- This widespread adoption is further supported by a mature healthcare IT infrastructure, broad availability of electronic health records (EHRs), and robust collaboration between academic institutions and AI startups, making North America a global leader in transforming clinical research operations through AI
U.S. AI-Powered Clinical Research Platform Market Insight
The U.S. AI-powered clinical research platform market captured the largest revenue share of 87% in 2024 within North America, fueled by rapid digitalization in healthcare, large-scale clinical trial activity, and federal initiatives supporting AI in life sciences. U.S.-based sponsors are increasingly leveraging AI platforms for site selection, automated patient matching, and decentralized trial management. The presence of companies such as IBM Watson Health, Medidata, and IQVIA is accelerating adoption across major academic medical centers and CROs.
Europe AI-Powered Clinical Research Platform Market Insight
The Europe AI-powered clinical research platform market is projected to expand at a CAGR of 19.7% during 2025 to 2032, driven by increasing demand for data transparency, regulatory compliance, and cost-effective drug development. European agencies are encouraging the integration of AI tools to streamline the approval process and improve trial efficiency. Cross-border data-sharing frameworks and large-scale biobanks across the region are strengthening the AI ecosystem for clinical trials.
U.K. AI-Powered Clinical Research Platform Market Insight
The U.K. AI-powered clinical research platform market is anticipated to grow at a noteworthy CAGR, supported by the government’s long-term investment in life sciences and AI innovation hubs such as the NHS AI Lab. With a strong digital health framework and access to centralized medical records, the U.K. is emerging as a preferred destination for real-world data-based trials and AI-driven protocol modeling.
Germany AI-Powered Clinical Research Platform Market Insight
The Germany AI-powered clinical research platform market is expanding steadily, driven by the country’s emphasis on high clinical trial standards and growing adoption of digital health tools. Germany's large healthcare dataset networks and AI-focused R&D investments, particularly in oncology and rare disease trials, support robust market growth. Regulatory initiatives encouraging safe and ethical AI use in healthcare further bolster platform deployment.
Asia-Pacific AI-Powered Clinical Research Platform Market Insight
The Asia-Pacific AI-powered clinical research platform market is poised to grow at the fastest CAGR of 19.3% from 2025 to 2032, propelled by growing clinical trial outsourcing, increasing digitization in healthcare, and rising pharmaceutical R&D expenditure in countries such as China, India, and Japan. Regional governments are also investing in cloud infrastructure and patient data repositories to support AI integration into trial workflows.
Japan AI-Powered Clinical Research Platform Market Insight
The Japan AI-powered clinical research platform market is gaining momentum due to the country's strong biotech sector and focus on personalized medicine. Japanese institutions are early adopters of AI in clinical trial simulation, protocol optimization, and patient engagement, especially in oncology and neurodegenerative studies. Government support for digital therapeutics and AI innovation also contributes to strong market expansion.
China AI-Powered Clinical Research Platform Market Insight
The China AI-powered clinical research platform market accounted for the largest market revenue share in Asia Pacific in 2024, supported by the nation's aggressive digital health policies and a rapidly expanding pharmaceutical industry. China is witnessing a surge in AI-driven drug discovery, and clinical trial management platforms are benefiting from large national health data sets and favorable government-backed digital transformation initiatives.
AI-Powered Clinical Research Platform Market Share
The AI-powered clinical research platform industry is primarily led by well-established companies, including:
- IBM Watson Health (U.S.)
- Oracle Health Sciences (U.S.)
- Medidata Solutions (U.S.)
- IQVIA (U.S.)
- Phesi Inc. (U.S.)
- Saama Technologies (U.S.)
- Concerto HealthAI (U.S.)
- Deep 6 AI (U.S.)
- Owkin (France)
- Antidote Technologies (U.K.)
- Unlearn.AI (U.S.)
- Tempus (U.S.)
- Trials.ai (U.S.)
- Standigm (South Korea)
- Biofourmis (Singapore)
- Cloud Pharmaceuticals (U.S.)
- Sensyne Health (U.K.)
- HealthVerity (U.S.)
- GNS Healthcare (U.S.)
- AiCure (U.S.)
Latest Developments in Global AI-Powered Clinical Research Platform Market
- In May 2025, PhaseV secured a USD 50M Series A funding round and announced a partnership with Alimentiv, a GI-focused CRO, to expand use of its ClinOps AI platform. The platform aims to modernize trial operations by providing precision-guided site selection and dynamic performance monitoring, reducing trial costs and timelines
- In June 2025, IQVIA launched custom-built AI agents in collaboration with NVIDIA, leveraging technologies such as NVIDIA NeMo and DGX Cloud. These agents are designed to enhance workflows across life sciences and healthcare data analysis platforms, with market availability expected later in 2025
- In March 2025, TrialX showcased its AI‑powered patient recruitment innovations at the Patients as Partners conference. Their generative AI tools can create protocol documents and multilingual trial materials in less than a day, significantly optimizing patient engagement and trial setup .
- In January 2025, Dash Bio, a biotech automation startup, raised USD11M in seed funding to develop its AI-powered robotics platform for analyzing clinical trial samples. This platform automates lab procedures to accelerate drug development with faster, higher-quality results
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.