Global Allergy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
23.05 Billion
USD
38.24 Billion
2024
2032
USD
23.05 Billion
USD
38.24 Billion
2024
2032
| 2025 –2032 | |
| USD 23.05 Billion | |
| USD 38.24 Billion | |
|
|
|
|
Allergy Treatment Market Size
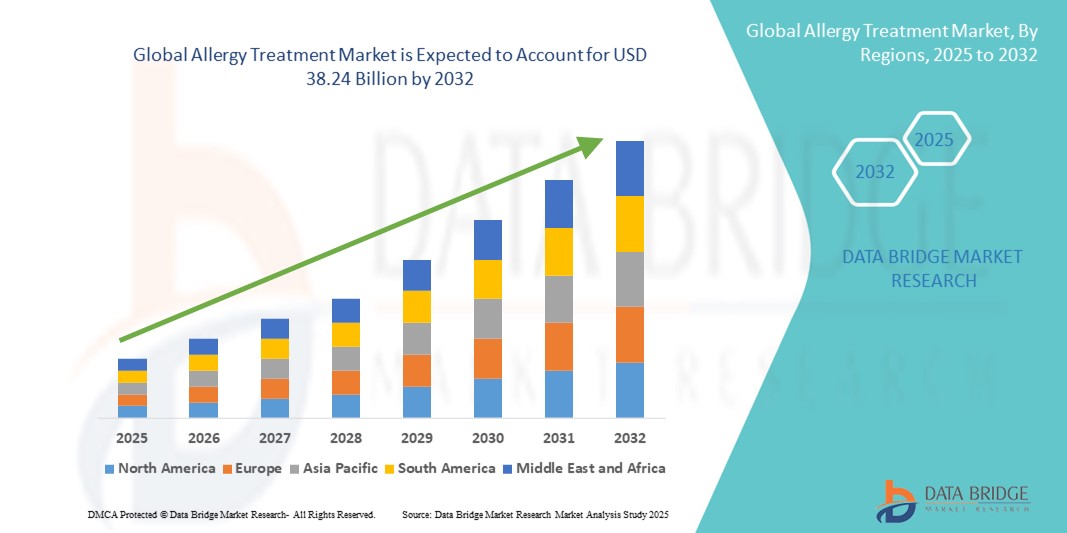
- The global allergy treatment market size was valued at USD 23.05 billion in 2024 and is expected to reach USD 38.24 billion by 2032, at a CAGR of 6.53% during the forecast period
- The market growth is largely fueled by the increasing prevalence of allergic conditions—such as allergic rhinitis, asthma, and food allergies—driven by urbanization, environmental pollution, and changing lifestyles across global populations
- Furthermore, advancements in immunotherapy, biologics, and personalized medicine are enhancing treatment options, leading to rising clinician confidence and patient adoption of cutting-edge allergy treatments
Allergy Treatment Market Analysis
- The global allergy treatment market is experiencing substantial growth, primarily driven by the increasing prevalence of allergic disorders such as asthma, allergic rhinitis, food allergies, eczema, and drug allergies. This surge is further supported by rising public health awareness regarding early diagnosis and appropriate treatment regimens, as well as growing concerns about environmental pollution and its impact on allergy development
- The demand for allergy treatments is being significantly propelled by the rising utilization of immunotherapy (allergy shots and sublingual tablets), second-generation antihistamines, intranasal corticosteroids, and biologics such as monoclonal antibodies. In addition, technological innovations in allergy diagnostics and drug delivery systems are enhancing treatment effectiveness and patient compliance
- North America dominated the allergy treatment market with the largest revenue share of 38.5% in 2024, largely due to high healthcare expenditure, widespread health insurance coverage, advanced allergy testing infrastructure, and the strong presence of major pharmaceutical and biotechnology firms developing targeted therapies. The U.S., in particular, is witnessing a surge in allergy treatment adoption across both urban and rural healthcare settings, driven by supportive government initiatives and continuous R&D investments
- Asia-Pacific is projected to be the fastest growing region in the allergy treatment market during the forecast period, attributed to rapid urbanization, increasing industrial pollution, rising disposable incomes, and expanding access to quality healthcare. Countries such as China, India, and Japan are seeing a sharp rise in cases of respiratory and skin allergies, prompting greater adoption of both prescription and over-the-counter allergy medications
- The anti-allergy drugs segment dominated the allergy treatment market with a share of 71.4% in 2024, as these medications are readily available, cost-effective, and suitable for managing mild to moderate allergy symptoms. These include antihistamines, decongestants, corticosteroids, and mast cell stabilizers
Report Scope and Allergy Treatment Market Segmentation
|
Attributes |
Allergy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Allergy Treatment Market Trends
Enhanced Convenience Through AI and Digital Integration in Allergy Treatment
- A significant and accelerating trend in the global allergy treatment market is the growing integration of artificial intelligence (AI), machine learning algorithms, and digital health platforms into various aspects of allergy care, including diagnosis, symptom tracking, and treatment optimization. These innovations are significantly improving the convenience, personalization, and accessibility of allergy treatments
- For instance, AI-enabled diagnostic platforms can analyze patient health records, environmental factors, and symptom patterns to aid in the early identification of allergies—be it food allergies, drug hypersensitivities, or airborne allergens such as pollen and dust mites. This streamlines the diagnostic process and enhances accuracy
- Digital applications powered by AI are becoming essential tools for allergy sufferers, offering real-time allergen exposure forecasts, personalized symptom tracking, medication reminders, and adaptive management plans. These features not only improve adherence to treatment but also empower patients to manage their condition proactively
- Voice-assisted technologies, integrated with platforms such as Amazon Alexa and Google Assistant, are further enhancing user convenience by allowing patients to set reminders, log symptoms, or access treatment information using hands-free voice commands. This is especially helpful for individuals managing chronic allergic conditions on a daily basis
- In addition, AI-driven telehealth platforms now enable allergy specialists to remotely monitor patient progress and adjust therapies in real-time. This reduces the need for frequent in-person visits, making care more accessible and reducing overall healthcare costs
- The convergence of AI, voice-enabled tools, and remote care platforms is reshaping the allergy treatment landscape, leading to more efficient, personalized, and patient-centric care models. As a result, healthcare providers and companies are increasingly investing in intelligent digital ecosystems that support end-to-end allergy care management
Allergy Treatment Market Dynamics
Driver
Rising Prevalence of Allergic Conditions and Increased Awareness Drive Market Growth
- The growing global prevalence of various allergic conditions—including allergic rhinitis, asthma, food allergies, and drug allergies—is one of the primary drivers fueling the demand for allergy treatment solutions across healthcare systems. This trend is further amplified by increasing awareness of allergy symptoms and the availability of advanced treatment options among patients and healthcare providers
- For instance, in April 2024, Alladapt Immunotherapeutics Inc. announced promising clinical data for ADP101, its multi-food oral immunotherapy treatment. Such innovations by leading biopharmaceutical companies are accelerating the development and commercialization of novel therapies in the Allergy Treatment market
- Changing environmental conditions, increased pollution levels, and rising exposure to allergens due to urbanization are contributing to the surge in allergic disorders globally. This has led to a significant uptick in medical consultations and prescriptions for antihistamines, corticosteroids, decongestants, and allergen-specific immunotherapy
- In addition, public and private health organizations are conducting large-scale campaigns to educate people about allergy management and prevention strategies. These efforts are improving diagnosis rates and treatment adherence, further contributing to market expansion
- The convenience of modern treatment options such as sublingual immunotherapy (SLIT), self-administered epinephrine auto-injectors, and long-acting biologics is also attracting patient attention, particularly in developed markets. The trend toward personalized allergy treatment plans, supported by improved diagnostic tools such as skin prick testing and IgE blood testing, is significantly enhancing treatment efficacy
- Restraint/Challenge
Limited Access and High Cost of Advanced Allergy Treatments Pose Market Barriers
- A major challenge in the Allergy Treatment market is the unequal access to advanced therapies due to cost constraints, particularly in low- and middle-income countries. Biologic therapies and immunotherapy options, while effective, often come with high price tags, limiting their affordability and widespread adoption
- For instance, monoclonal antibody treatments such as omalizumab and dupilumab, although offering superior outcomes for severe allergies and asthma, remain financially out of reach for many patients due to limited insurance coverage and high out-of-pocket costs
- Further complicating access is the shortage of trained allergy specialists in several regions, particularly in rural and underserved areas. This hampers early diagnosis and appropriate treatment, resulting in under-treatment or mismanagement of allergic conditions
- Another key restraint is patient hesitancy toward long-term immunotherapy due to the need for repeated administration, potential side effects, and the extended duration of treatment. This lack of immediate relief compared to symptomatic treatments such as antihistamines can deter patient adherence
- Overcoming these limitations will require increased investment in healthcare infrastructure, the development of cost-effective treatment alternatives, and robust healthcare reimbursement policies. In addition, raising awareness about the long-term benefits of allergy immunotherapy through clinician and patient education can help improve acceptance and adherence
Allergy Treatment Market Scope
The market is segmented on the basis of type, treatment, route of administration, end-users, and distribution channel.
- By Type
On the basis of type, the allergy treatment market is segmented into eye allergy, food allergy, skin allergy, asthma, rhinitis, and others. The asthma segment accounted for the largest revenue share of 34.6% in 2024, owing to the increasing prevalence of allergic asthma triggered by pollution, dust mites, and environmental allergens. The rising urban population and deteriorating air quality in both developed and developing nations are key drivers. The rhinitis segment also holds a significant market share due to seasonal pollen allergies and increased sensitivity to airborne particles.
The skin allergy segment is the fastest growing segment with a CAGR of 12% due to heightened awareness, improved dermatological diagnostics, and growing use of allergen-containing products. Food allergies, especially among children, are on the rise, prompting growth in this subsegment. Overall, growing allergy awareness, better diagnosis, and rising environmental triggers are contributing to growth across all types.
- By Treatment
On the basis of treatment, the allergy treatment market is segmented into anti-allergy drugs and immunotherapy. The anti-allergy drugs segment held the largest market share of 71.4% in 2024, as these medications are readily available, cost-effective, and suitable for managing mild to moderate allergy symptoms. These include antihistamines, decongestants, corticosteroids, and mast cell stabilizers.
Their wide availability in retail and online pharmacies further boosts accessibility. However, the immunotherapy segment is projected to grow at a CAGR of 8.5% from 2025 to 2032, driven by increasing patient preference for long-term, disease-modifying treatments. This includes allergy shots and sublingual tablets that provide sustained relief by desensitizing the immune system. As allergic conditions become chronic, immunotherapy adoption is steadily rising.
- By Route of Administration
On the basis of route of administration, the allergy treatment market is segmented into oral, inhalation, intranasal, and others. The oral segment led the market with a share of 45.2% in 2024, supported by patient preference for easy and non-invasive treatment forms such as tablets and syrups. These are typically the first-line therapy for allergy management and widely distributed.
The inhalation segment is expanding at a CAGR of 7.2%, primarily due to the increasing prevalence of allergic asthma and advances in inhaler technology. Intranasal routes are particularly effective in rhinitis and sinus-related allergies, offering rapid local relief with minimal side effects. Routes such as subcutaneous injections are also gaining popularity under the immunotherapy segment. Convenience, compliance, and innovation in drug delivery devices are key growth enablers across all routes.
- By End-Users
On the basis of end-users, the allergy treatment market is segmented into hospitals, specialty clinics, homecare, and others. The hospital segment captured the largest share of 39.5% in 2024, as hospitals are equipped with advanced facilities for allergy testing, emergency care, and specialist consultations. In severe or life-threatening allergy cases, such as anaphylaxis, immediate hospital-based treatment is crucial. Specialty clinics cater to long-term management, especially immunotherapy, and are becoming preferred centers for tailored allergy care.
Homecare is witnessing fast growth with a CAGR of 8.1%, driven by increased patient awareness and adoption of home-based solutions such as OTC antihistamines, nebulizers, and teleconsultations. Patients with mild to moderate symptoms prefer convenient, self-managed options. This shift reflects the rising importance of decentralized care and remote monitoring.
- By Distribution Channel
On the basis of distribution channel, the allergy treatment market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The retail pharmacy segment held the highest market share of 47.6% in 2024, as it offers quick access to both prescription and non-prescription allergy drugs. Chain pharmacies, local drugstores, and over-the-counter sales drive this segment.
The online pharmacy segment is expected to grow at a CAGR of 9.4%, supported by increasing digital health adoption, convenience, and availability of discounted rates. Online platforms also enable auto-refill services and doorstep delivery, making them popular among chronic allergy patients. Hospital pharmacies are critical for supplying specialty treatments, especially in immunotherapy and emergency care. The diversification of distribution channels ensures wider reach and accessibility of treatments across urban and rural populations.
Allergy Treatment Market Regional Analysis
- North America dominated the allergy treatment market with the largest revenue share of 38.5% in 2024, driven by the rising prevalence of allergic conditions such as allergic rhinitis, asthma, eczema, and food allergies
- The region's strong healthcare infrastructure, increased awareness about allergic diseases, and wide availability of advanced treatment options such as immunotherapy, antihistamines, corticosteroids, and biologics have significantly contributed to market growth
- Furthermore, the presence of leading pharmaceutical companies, ongoing research into personalized allergy treatments, and favorable reimbursement policies are enhancing patient access to innovative therapies across both pediatric and adult populations
U.S. Allergy Treatment Market Insight
The U.S. allergy treatment market captured the largest revenue share of 81% in 2024 within North America, supported by the high incidence of seasonal allergies and food-related hypersensitivities. The country is witnessing growing demand for sublingual immunotherapy and biologic drugs for severe allergic asthma and atopic dermatitis. Technological advancements in diagnostic tools, coupled with increased adoption of at-home allergy testing kits and digital health platforms, are enhancing patient engagement and management. In addition, strong R&D investment and FDA approvals for novel allergy medications are further driving market expansion in the U.S.
Europe Allergy Treatment Market Insight
The Europe allergy treatment market is projected to expand at a substantial CAGR throughout the forecast period, fueled by the growing burden of allergic diseases and increasing awareness about early diagnosis and treatment. Rising environmental pollution, climate change, and urban lifestyle factors are contributing to the surge in allergy cases across the region. Government initiatives focused on allergy prevention, expanding access to immunotherapy, and investment in clinical trials for novel drugs are significantly supporting the market. Moreover, strong collaborations between academic institutions and biotech firms are helping introduce more effective and safer allergy treatments.
U.K. Allergy Treatment Market Insight
The U.K. allergy treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the rise in allergic rhinitis, asthma, and food allergies among children and adults. Growing awareness campaigns by organizations such as Allergy UK and National Health Service (NHS) programs focusing on allergy education and patient care are improving diagnosis rates. Moreover, the adoption of biologics and the expansion of specialized allergy clinics are enhancing treatment accessibility. The increasing focus on research into food allergy desensitization therapies is also expected to stimulate long-term market growth.
Germany Allergy Treatment Market Insight
The Germany allergy treatment market is expected to expand at a considerable CAGR during the forecast period, driven by strong government support for allergy prevention and research, and increasing demand for targeted therapies. Germany’s well-established healthcare system and widespread insurance coverage for allergy diagnostics and treatment are encouraging more patients to seek medical help. Rising adoption of immunotherapy for hay fever, allergic asthma, and insect venom allergies, along with the availability of minimally invasive diagnostic tools, are further fueling the market. Increased funding for academic research into environmental allergens also supports innovation in this field.
Asia-Pacific Allergy Treatment Market Insight
The Asia-Pacific allergy treatment market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, owing to rapid urbanization, increasing pollution levels, changing dietary habits, and rising awareness of allergic conditions. Countries such as China, Japan, and India are witnessing a spike in allergic diseases, including asthma, allergic rhinitis, and food allergies. Government health programs aimed at improving access to healthcare, coupled with the rising penetration of private allergy clinics and e-health services, are promoting early diagnosis and treatment. Moreover, growing investments by pharmaceutical companies in APAC are expanding the availability of advanced allergy therapies.
Japan Allergy Treatment Market Insight
The Japan allergy treatment market is gaining momentum due to the high prevalence of pollen-related allergies, rising pollution levels, and an aging population prone to chronic allergic conditions. Innovations in allergy diagnostics, such as component-resolved diagnostics (CRD), and the development of targeted biologics for conditions such as eosinophilic asthma are contributing to market growth. In addition, public health initiatives focused on hay fever and the availability of over-the-counter allergy medications are increasing consumer awareness and accessibility to treatment. The demand for personalized allergy management solutions is also growing steadily in Japan.
China Allergy Treatment Market Insight
The China allergy treatment market accounted for the largest revenue share in the Asia-Pacific region in 2024, driven by rapid urbanization, industrialization, and lifestyle changes that have led to a rise in allergic diseases. China’s expanding healthcare infrastructure, increasing awareness about allergies, and the growing middle-class population are major factors propelling market growth. Domestic and international pharmaceutical companies are actively launching new drugs and therapies in the region, including traditional Chinese medicine-based formulations for allergy relief. Government-backed allergy awareness campaigns and improvements in allergy testing availability are further accelerating the market in China.
Allergy Treatment Market Share
The allergy treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Viatris Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Boehringer Ingelheim International GmbH (Germany)
- AstraZeneca (U.K.)
- Johnson & Johnson and its affiliates (U.S.)
- Bayer AG (Germany)
- Merck & Co., Inc. (U.S.)
- Prestige Consumer Healthcare Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Almirall, S.A (Spain)
- Zenomed Healthcare Private Limited (India)
- Cadila Pharmaceuticals (India)
- Astellas Pharma Inc. (Japan)
- Lilly (U.S.)
Latest Developments in Global Allergy Treatment Market
- In April 2025, Sanofi and Regeneron announced that the U.S. Food and Drug Administration (FDA) approved Dupixent (dupilumab) for treating chronic spontaneous urticaria (CSU) in patients aged 12 and older. This marks the first new targeted therapy approved for CSU in more than a decade, offering significant symptom relief across overlapping atopic conditions such as asthma and atopic dermatitis
- In July 2024, the FDA expanded the age eligibility for Palforzia, the first FDA-approved oral immunotherapy for peanut allergy, to include toddlers aged 1 through 3 years. Previously limited to ages 4–17, this expansion represents a major milestone for early intervention and broader accessibility of peanut desensitization therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ALLERGY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ALLERGY TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ALLERGY TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S 5 FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.1.1 PREVALENCE OF ASTHMA
5.1.2 PREVALENCE OF VENOM ALLERGY
5.1.3 PREVALENCE OF ALLERGIC RHINITIS
5.1.4 PREVALENCE OF CONJUNCTIVITIS
5.1.5 PREVALENCE OF ATOPIC DERMATITIS
5.1.6 PREVALENCE OF URTICARIA
5.1.7 PREVALENCE OF ANAPHYLAXIS
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH DOCTORS
6.8 INTERVIEWS WITH SALES EXECUTIVES
6.9 INTERVIEWS WITH PHARMACIES
6.1 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
7.1 FDA APPROVALS
7.2 EMA APPROVALS
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKET OVERVIEW
10.1 DRIVERS
10.2 RESTRAINTS
10.3 OPPORTUNITIES
10.4 CHALLENGES
11 GLOBAL ALLERGY TREATMENT MARKET, BY TREATMENT
11.1 OVERVIEW
11.2 MEDICATION
11.2.1 CORTICOSTEROIDS
11.2.1.1. BY DRUGS
11.2.1.1.1. BUDESONIDE
11.2.1.1.1.1 MARKET VALUE (USD MN)
11.2.1.1.1.2 MAKET VOLUME (IU)
11.2.1.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.2. FULTICASONE FUROATE
11.2.1.1.2.1 MARKET VALUE (USD MN)
11.2.1.1.2.2 MAKET VOLUME (IU)
11.2.1.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.3. FLUTICASONE PROPIONATE
11.2.1.1.3.1 MARKET VALUE (USD MN)
11.2.1.1.3.2 MAKET VOLUME (IU)
11.2.1.1.3.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.4. MOMETASONE
11.2.1.1.4.1 MARKET VALUE (USD MN)
11.2.1.1.4.2 MAKET VOLUME (IU)
11.2.1.1.4.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.5. TRIAMCINOLONE
11.2.1.1.5.1 MARKET VALUE (USD MN)
11.2.1.1.5.2 MAKET VOLUME (IU)
11.2.1.1.5.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.6. BECLOMETHASONE
11.2.1.1.6.1 MARKET VALUE (USD MN)
11.2.1.1.6.2 MAKET VOLUME (IU)
11.2.1.1.6.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.7. CICLESONIDE
11.2.1.1.7.1 MARKET VALUE (USD MN)
11.2.1.1.7.2 MAKET VOLUME (IU)
11.2.1.1.7.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.8. FLUOROMETHOLONE
11.2.1.1.8.1 MARKET VALUE (USD MN)
11.2.1.1.8.2 MAKET VOLUME (IU)
11.2.1.1.8.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.9. PREDNISOLONE
11.2.1.1.9.1 MARKET VALUE (USD MN)
11.2.1.1.9.2 MAKET VOLUME (IU)
11.2.1.1.9.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.10. PREDNISONE
11.2.1.1.10.1 MARKET VALUE (USD MN)
11.2.1.1.10.2 MAKET VOLUME (IU)
11.2.1.1.10.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.11. METHYLPREDNISOLONE
11.2.1.1.11.1 MARKET VALUE (USD MN)
11.2.1.1.11.2 MAKET VOLUME (IU)
11.2.1.1.11.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.12. BETAMETHASONE
11.2.1.1.12.1 MARKET VALUE (USD MN)
11.2.1.1.12.2 MAKET VOLUME (IU)
11.2.1.1.12.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.13. DESONIDE
11.2.1.1.13.1 MARKET VALUE (USD MN)
11.2.1.1.13.2 MAKET VOLUME (IU)
11.2.1.1.13.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.14. HYDROCORTISONE
11.2.1.1.14.1 MARKET VALUE (USD MN)
11.2.1.1.14.2 MAKET VOLUME (IU)
11.2.1.1.14.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.15. OTHERS
11.2.1.2. BY ROUTE OF ADMINISTRATIONS
11.2.1.2.1. ORAL
11.2.1.2.1.1 PILLS
11.2.1.2.1.2 TABLETS
11.2.1.2.1.3 CAPSULES
11.2.1.2.1.4 OTHERS
11.2.1.2.2. TOPICALS
11.2.1.2.2.1 GELS
11.2.1.2.2.2 NASAL SPRAYS
11.2.1.2.2.3 EYE DROPS
11.2.1.2.2.4 CREAM
11.2.1.2.2.5 OTHERS
11.2.1.2.3. PARENTERAL
11.2.1.2.3.1 SOLUTIONS
11.2.1.2.3.2 SUSPENTIONS
11.2.1.2.3.3 OTHERS
11.2.1.3. BY DRUG TYPE
11.2.1.3.1. BRANDED
11.2.1.3.2. GENERICS
11.2.2 ANTIHISTAMINES
11.2.2.1. BY DRUGS
11.2.2.1.1. IORATADINE
11.2.2.1.1.1 MARKET VALUE (USD MN)
11.2.2.1.1.2 MAKET VOLUME (IU)
11.2.2.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.2. CETIRIZINE
11.2.2.1.2.1 MARKET VALUE (USD MN)
11.2.2.1.2.2 MAKET VOLUME (IU)
11.2.2.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.3. DIPHENHYDRAMINE
11.2.2.1.3.1 MARKET VALUE (USD MN)
11.2.2.1.3.2 MAKET VOLUME (IU)
11.2.2.1.3.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.4. CHLORPHENIRAMINE
11.2.2.1.4.1 MARKET VALUE (USD MN)
11.2.2.1.4.2 MAKET VOLUME (IU)
11.2.2.1.4.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.5. LEVOCETIRIZINE
11.2.2.1.5.1 MARKET VALUE (USD MN)
11.2.2.1.5.2 MAKET VOLUME (IU)
11.2.2.1.5.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.6. FEXOFENADINE
11.2.2.1.6.1 MARKET VALUE (USD MN)
11.2.2.1.6.2 MAKET VOLUME (IU)
11.2.2.1.6.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.7. DESLORATADINE
11.2.2.1.7.1 MARKET VALUE (USD MN)
11.2.2.1.7.2 MAKET VOLUME (IU)
11.2.2.1.7.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.8. KETOTIFEN
11.2.2.1.8.1 MARKET VALUE (USD MN)
11.2.2.1.8.2 MAKET VOLUME (IU)
11.2.2.1.8.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.9. OLOPATADINE
11.2.2.1.9.1 MARKET VALUE (USD MN)
11.2.2.1.9.2 MAKET VOLUME (IU)
11.2.2.1.9.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.10. PHENIRAMINE
11.2.2.1.10.1 MARKET VALUE (USD MN)
11.2.2.1.10.2 MAKET VOLUME (IU)
11.2.2.1.10.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.11. AZELASTINE
11.2.2.1.11.1 MARKET VALUE (USD MN)
11.2.2.1.11.2 MAKET VOLUME (IU)
11.2.2.1.11.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.12. OTHERS
11.2.2.2. BY ROUTE OF ADMINISTRATIONS
11.2.2.2.1. ORAL
11.2.2.2.1.1 PILLS
11.2.2.2.1.2 TABLETS
11.2.2.2.1.3 CAPSULES
11.2.2.2.1.4 OTHERS
11.2.2.2.2. TOPICALS
11.2.2.2.2.1 GELS
11.2.2.2.2.2 NASAL SPRAYS
11.2.2.2.2.3 EYE DROPS
11.2.2.2.2.4 CREAM
11.2.2.2.2.5 OTHERS
11.2.2.2.3. PARENTERAL
11.2.2.2.3.1 SOLUTIONS
11.2.2.2.3.2 SUSPENTIONS
11.2.2.2.3.3 OTHERS
11.2.2.3. BY DRUG TYPE
11.2.2.3.1. BRANDED
11.2.2.3.2. GENERICS
11.2.3 DECONGESTANTS
11.2.3.1. BY DRUGS
11.2.3.1.1. PSEDOEPHRINE
11.2.3.1.1.1 MARKET VALUE (USD MN)
11.2.3.1.1.2 MARKET VOLUME (SU)
11.2.3.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.2. PHENYLEPHRINE
11.2.3.1.2.1 MARKET VALUE (USD MN)
11.2.3.1.2.2 MARKET VOLUME (SU)
11.2.3.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.3. OXYMETAZOLINE
11.2.3.1.3.1 MARKET VALUE (USD MN)
11.2.3.1.3.2 MARKET VOLUME (SU)
11.2.3.1.3.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.4. PSEUDOEPHEDRINE
11.2.3.1.4.1 MARKET VALUE (USD MN)
11.2.3.1.4.2 MARKET VOLUME (SU)
11.2.3.1.4.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.5. TETRAHYDROZOLINE
11.2.3.1.5.1 MARKET VALUE (USD MN)
11.2.3.1.5.2 MAKET VOLUME (IU)
11.2.3.1.5.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.6. OTHERS
11.2.3.2. BY ROUTE OF ADMINISTRATIONS
11.2.3.2.1. ORAL
11.2.3.2.1.1 PILLS
11.2.3.2.1.2 TABLETS
11.2.3.2.1.3 CAPSULES
11.2.3.2.1.4 OTHERS
11.2.3.2.2. TOPICALS
11.2.3.2.2.1 GELS
11.2.3.2.2.2 NASAL SPRAYS
11.2.3.2.2.3 EYE DROPS
11.2.3.2.2.4 CREAM
11.2.3.2.2.5 OTHERS
11.2.3.2.3. PARENTERAL
11.2.3.2.3.1 SOLUTIONS
11.2.3.2.3.2 SUSPENTIONS
11.2.3.2.3.3 OTHERS
11.2.3.3. BY DRUG TYPE
11.2.3.3.1. BRANDED
11.2.3.3.2. GENERICS
11.3 MAST CELL STABILIZERS
11.3.1.1. BY DRUGS
11.3.1.1.1. CROMOLYN
11.3.1.1.1.1 MARKET VALUE (USD MN)
11.3.1.1.1.2 MAKET VOLUME (IU)
11.3.1.1.1.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.2. LODOXAMIDE
11.3.1.1.2.1 MARKET VALUE (USD MN)
11.3.1.1.2.2 MAKET VOLUME (IU)
11.3.1.1.2.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.3. NEDOCROMIL
11.3.1.1.3.1 MARKET VALUE (USD MN)
11.3.1.1.3.2 MAKET VOLUME (IU)
11.3.1.1.3.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.4. OTHERS
11.3.1.2. BY DRUG TYPE
11.3.1.2.1. GENERICS
11.3.1.2.2. BRANDED
11.3.1.3. BY ROUTE OF ADMINISTRATIONS
11.3.1.3.1. ORAL
11.3.1.3.1.1 PILLS
11.3.1.3.1.2 TABLETS
11.3.1.3.1.3 CAPSULES
11.3.1.3.1.4 OTHERS
11.3.1.3.2. TOPICALS
11.3.1.3.2.1 GELS
11.3.1.3.2.2 NASAL SPRAYS
11.3.1.3.2.3 EYE DROPS
11.3.1.3.2.4 CREAM
11.3.1.3.2.5 OTHERS
11.3.1.3.3. PARENTERAL
11.3.1.3.3.1 SOLUTIONS
11.3.1.3.3.2 SUSPENTIONS
11.3.1.3.3.3 OTHERS
11.3.1.4. BY DRUG TYPE
11.3.1.4.1. BRANDED
11.3.1.4.2. GENERICS
11.4 EPINEPHRINE SHOTS
11.4.1.1. ADRENACLICK
11.4.1.1.1. MARKET VALUE (USD MN)
11.4.1.1.2. MAKET VOLUME (IU)
11.4.1.1.3. AVERAGE SELLING PRICE (USD)
11.4.1.2. AUVI-Q
11.4.1.2.1. MARKET VALUE (USD MN)
11.4.1.2.2. MAKET VOLUME (IU)
11.4.1.2.3. AVERAGE SELLING PRICE (USD)
11.4.1.3. EPIPEN
11.4.1.3.1. MARKET VALUE (USD MN)
11.4.1.3.2. MAKET VOLUME (IU)
11.4.1.3.3. AVERAGE SELLING PRICE (USD)
11.4.1.4. EPIPEN JR
11.4.1.4.1. MARKET VALUE (USD MN)
11.4.1.4.2. MAKET VOLUME (IU)
11.4.1.4.3. AVERAGE SELLING PRICE (USD)
11.4.1.5. OTHERS
11.4.2 LEUKOTRIENE INHIBITORS
11.4.2.1. BY DRUGS
11.4.2.1.1. MOTELUKAST
11.4.2.1.1.1 MARKET VALUE (USD MN)
11.4.2.1.1.2 MAKET VOLUME (IU)
11.4.2.1.1.3 AVERAGE SELLING PRICE (USD)
11.4.2.1.2. ZAFIRLUKAST
11.4.2.1.2.1 MARKET VALUE (USD MN)
11.4.2.1.2.2 MAKET VOLUME (IU)
11.4.2.1.2.3 AVERAGE SELLING PRICE (USD)
11.4.2.1.3. OTHERS
11.4.2.2. BY DRUG TYPE
11.4.2.2.1. BRANDED
11.4.2.2.2. GENERICS
11.4.3 OTHERS
11.5 IMMUNOTHERAPY
11.5.1 SUBLINGUAL IMMUNOTHERAPY (SLIT)
11.5.2 SUBCUTANEOUS IMMUNOTHERAPY (SCIT)
12 GLOBAL ALLERGY TREATMENT MARKET, BY DISEASE TYPE
12.1 OVERVIEW
12.2 FOOD ALLERGIES
12.2.1 MILK ALLERGY
12.2.2 EGG ALLLERGY
12.2.3 FISH ALLERGY
12.2.4 PEANUTS ALLERGY
12.2.5 WHEAT ALLERGY
12.2.6 SOYABEAN ALLERGY
12.2.7 SEASAME ALLERGY
12.2.8 TREE NUT ALLERGY
12.2.9 CRUSTACEAN SHELLFISH ALLERGY
12.2.10 OTHERS
12.3 DRUG ALLERGY
12.3.1 PENICILLIN ALLERGY
12.3.2 SULFONAMIDES ALLERGY
12.3.3 ANTICONVULSANT ALLERGY
12.3.4 NSAIDS ALLERGY
12.3.5 CHEMOTHERAPY ALLERGY
12.3.6 OTHERS
12.4 PET ALLERGY
12.4.1 CATS ALLERGY
12.4.2 DOG ALLERGY
12.4.3 OTYHERS
12.5 INSECT ALLERGY
12.5.1 MOSQUITOES ALLERGY
12.5.2 KISSING BUGS ALLERGY
12.5.3 BED BUGS ALLERGY
12.5.4 FLEAS ALLERGY
12.5.5 OTHERS
12.6 LATEX ALLERGY
12.6.1 IMMUNOGLOBULIN E (IGE ) MEDIATED
12.6.2 CONTACT DERMATITIS
12.7 MOLD ALLERGY
12.7.1 ALTERNARIA ALLERGY
12.7.2 ASPERGILLUS ALLERGY
12.7.3 CLADOSPORIUM ALLERGY
12.7.4 OTHERS
12.8 POLLEN ALLERGY
12.9 SKIN ALLERGY
12.9.1 ATOPIC DERMATITIS
12.9.2 URTICARIA
12.1 ASTHMA
12.11 ALLERGIC RHINITIS
12.12 CONJUNCTIVITIS
12.13 OTHERS
13 GLOBAL ALLERGY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 ORAL
13.2.1 PILLS
13.2.2 TABLETS
13.2.3 CAPSULES
13.2.4 OTHERS
13.3 TOPICALS
13.3.1 GELS
13.3.2 NASAL SPRAYS
13.3.3 EYE DROPS
13.3.4 CREAM
13.3.5 OTHERS
13.4 PARENTERAL
13.4.1 SOLUTIONS
13.4.2 SUSPENTIONS
13.4.3 OTHERS
14 GLOBAL ALLERGY TREATMENT MARKET, BY DRUG TYPE
14.1 BRANDED
14.2 GENERICS
15 GLOBAL ALLERGY TREATMENT MARKET, BY MODE OF PURCHASE
15.1 OVERVIEW
15.2 OVER THE COUNTER
15.3 PRESCRIPTION
16 GLOBAL ALLERGY TREATMENT MARKET, BY AGE GROUP
16.1 OVERVIEW
16.2 PEDIATRIC
16.3 ADULTS
16.4 GERIATRIC
17 GLOBAL ALLERGY TREATMENT MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITAL
17.3 CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 GLOBAL ALLERGY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACY
18.3 ONLINE PHARMACY
18.4 RETAIL PHARMACY
18.5 OTHERS
19 GLOBAL ALLERGY TREATMENT MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 MERGERS & ACQUISITIONS
19.3 NEW PRODUCT DEVELOPMENT & APPROVALS
19.4 EXPANSIONS
19.5 REGULATORY CHANGES
19.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL ALLERGY TREATMENT MARKET, BY GEOGRAPHY
20.1 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
20.2 NORTH AMERICA
20.2.1 U.S.
20.2.1.1. U.S. GOUT THERAPEUTICS MARKET, BY TREATMENT
20.2.1.2. U.S. GOUT THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION
20.2.1.3. U.S. GOUT THERAPEUTICS MARKET, BY END USER
20.2.1.4. U.S. GOUT THERAPEUTICS MARKET, BY DISTRIBUTION CHANNEL
20.3 CANADA
20.4 MEXICO
20.5 EUROPE
20.5.1 GERMANY
20.5.2 FRANCE
20.5.3 U.K.
20.5.4 FINLAND
20.5.5 DENMARK
20.5.6 NORWAY
20.5.7 POLAND
20.5.8 ITALY
20.5.9 SPAIN
20.5.10 RUSSIA
20.5.11 TURKEY
20.5.12 BELGIUM
20.5.13 NETHERLANDS
20.5.14 SWITZERLAND
20.5.15 SWEDEN
20.5.16 REST OF EUROPE
20.6 ASIA-PACIFIC
20.6.1 JAPAN
20.6.2 CHINA
20.6.3 SOUTH KOREA
20.6.4 INDIA
20.6.5 SINGAPORE
20.6.6 THAILAND
20.6.7 INDONESIA
20.6.8 MALAYSIA
20.6.9 PHILIPPINES
20.6.10 AUSTRALIA
20.6.11 NEW ZEALAND
20.6.12 VIETNAM
20.6.13 TAIWAN
20.6.14 REST OF ASIA-PACIFIC
20.6.15
20.7 SOUTH AMERICA
20.7.1 BRZIL
20.7.2 ARGENTINA
20.7.3 REST OF SOUTH AMERICA
20.8 MIDDLE EAST AND AFRICA
20.8.1 SOUTH AFRICA
20.8.2 SAUDI ARABIA
20.8.3 UAE
20.8.4 EGYPT
20.8.5 KUWAIT
20.8.6 OMAN
20.8.7 ISRAEL
20.8.8 BAHRAIN
20.8.9 REST OF MIDDLE EAST AND AFRICA
20.9 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
21 GLOBAL ALLERGY TREATMENT MARKET, SWOT AND DBMR ANALYSIS
22 GLOBAL ALLERGY TREATMENT MARKET, COMPANY PROFILE
22.1 SANOFI
22.1.1 COMPANY OVERVIEW
22.1.2 REVENUE ANALYSIS
22.1.3 GEOGRAPHIC PRESENCE
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 GLAXOSMITHKLINE PLC
22.2.1 COMPANY OVERVIEW
22.2.2 REVENUE ANALYSIS
22.2.3 GEOGRAPHIC PRESENCE
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 PFIZER INC.
22.3.1 COMPANY OVERVIEW
22.3.2 REVENUE ANALYSIS
22.3.3 GEOGRAPHIC PRESENCE
22.3.4 PRODUCT PORTFOLIO
22.3.5 RECENT DEVELOPMENTS
22.4 JANSSEN GLOBAL SERVICES, LLC.
22.4.1 COMPANY OVERVIEW
22.4.2 REVENUE ANALYSIS
22.4.3 GEOGRAPHIC PRESENCE
22.4.4 PRODUCT PORTFOLIO
22.4.5 RECENT DEVELOPMENTS
22.5 ABBVIE
22.5.1 COMPANY OVERVIEW
22.5.2 REVENUE ANALYSIS
22.5.3 GEOGRAPHIC PRESENCE
22.5.4 PRODUCT PORTFOLIO
22.5.5 RECENT DEVELOPMENTS
22.6 NOVARTIS AG
22.6.1 COMPANY OVERVIEW
22.6.2 REVENUE ANALYSIS
22.6.3 GEOGRAPHIC PRESENCE
22.6.4 PRODUCT PORTFOLIO
22.6.5 RECENT DEVELOPMENTS
22.7 LUPIN
22.7.1 COMPANY OVERVIEW
22.7.2 REVENUE ANALYSIS
22.7.3 GEOGRAPHIC PRESENCE
22.7.4 PRODUCT PORTFOLIO
22.7.5 RECENT DEVELOPMENTS
22.8 AMNEAL PHARMACEUTICALS LLC
22.8.1 COMPANY OVERVIEW
22.8.2 REVENUE ANALYSIS
22.8.3 GEOGRAPHIC PRESENCE
22.8.4 PRODUCT PORTFOLIO
22.8.5 RECENT DEVELOPMENTS
22.9 DR. REDDY’S LABORATORIES LTD.
22.9.1 COMPANY OVERVIEW
22.9.2 REVENUE ANALYSIS
22.9.3 GEOGRAPHIC PRESENCE
22.9.4 PRODUCT PORTFOLIO
22.9.5 RECENT DEVELOPMENTS
22.1 SUN PHARMACEUTICAL INDUSTRIES LTD.
22.10.1 COMPANY OVERVIEW
22.10.2 REVENUE ANALYSIS
22.10.3 GEOGRAPHIC PRESENCE
22.10.4 PRODUCT PORTFOLIO
22.10.5 RECENT DEVELOPMENTS
22.11 TEVA PHARMACEUTICAL INDUSTRIES LTD
22.11.1 COMPANY OVERVIEW
22.11.2 REVENUE ANALYSIS
22.11.3 GEOGRAPHIC PRESENCE
22.11.4 PRODUCT PORTFOLIO
22.11.5 RECENT DEVELOPMENTS
22.12 MYLAN N.V
22.12.1 COMPANY OVERVIEW
22.12.2 REVENUE ANALYSIS
22.12.3 GEOGRAPHIC PRESENCE
22.12.4 PRODUCT PORTFOLIO
22.12.5 RECENT DEVELOPMENTS
22.13 ZYDUS CADILA
22.13.1 COMPANY OVERVIEW
22.13.2 REVENUE ANALYSIS
22.13.3 GEOGRAPHIC PRESENCE
22.13.4 PRODUCT PORTFOLIO
22.13.5 RECENT DEVELOPMENTS
22.14 CIPLA
22.14.1 COMPANY OVERVIEW
22.14.2 REVENUE ANALYSIS
22.14.3 GEOGRAPHIC PRESENCE
22.14.4 PRODUCT PORTFOLIO
22.14.5 RECENT DEVELOPMENTS
22.15 GENENTECH, USA INC.
22.15.1 COMPANY OVERVIEW
22.15.2 REVENUE ANALYSIS
22.15.3 GEOGRAPHIC PRESENCE
22.15.4 PRODUCT PORTFOLIO
22.15.5 RECENT DEVELOPMENTS
22.16 STALLERGENES GREER
22.16.1 COMPANY OVERVIEW
22.16.2 REVENUE ANALYSIS
22.16.3 GEOGRAPHIC PRESENCE
22.16.4 PRODUCT PORTFOLIO
22.16.5 RECENT DEVELOPMENTS
22.17 ALK
22.17.1 COMPANY OVERVIEW
22.17.2 REVENUE ANALYSIS
22.17.3 GEOGRAPHIC PRESENCE
22.17.4 PRODUCT PORTFOLIO
22.17.5 RECENT DEVELOPMENTS
22.18 ALLERGY THERAPEUTICS
22.18.1 COMPANY OVERVIEW
22.18.2 REVENUE ANALYSIS
22.18.3 GEOGRAPHIC PRESENCE
22.18.4 PRODUCT PORTFOLIO
22.18.5 RECENT DEVELOPMENTS
22.19 ADAMIS PHARMACEUTICALS CORPORATION
22.19.1 COMPANY OVERVIEW
22.19.2 REVENUE ANALYSIS
22.19.3 GEOGRAPHIC PRESENCE
22.19.4 PRODUCT PORTFOLIO
22.19.5 RECENT DEVELOPMENTS
22.2 HOLLISTERSTIER ALLERGY. A JUBILANT PHARMA COMPANY
22.20.1 COMPANY OVERVIEW
22.20.2 REVENUE ANALYSIS
22.20.3 GEOGRAPHIC PRESENCE
22.20.4 PRODUCT PORTFOLIO
22.20.5 RECENT DEVELOPMENTS
22.21 HAL ALLERGY B.V.
22.21.1 COMPANY OVERVIEW
22.21.2 REVENUE ANALYSIS
22.21.3 GEOGRAPHIC PRESENCE
22.21.4 PRODUCT PORTFOLIO
22.21.5 RECENT DEVELOPMENTS
22.22 SUNOVION
22.22.1 COMPANY OVERVIEW
22.22.2 REVENUE ANALYSIS
22.22.3 GEOGRAPHIC PRESENCE
22.22.4 PRODUCT PORTFOLIO
22.22.5 RECENT DEVELOPMENTS
22.23 APOTEX CORPORATION
22.23.1 COMPANY OVERVIEW
22.23.2 PRODUCT PORTFOLIO
22.23.3 REVENUE ANALYSIS
22.23.4 GEOGRAPHIC PRESENCE
22.23.5 PRODUCT PORTFOLIO
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
23 RELATED REPORTS
24 CONCLUSION
25 QUESTIONNAIRE
26 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.