Global Alzheimers Anti Amyloid Monoclonal Antibodies Market
Market Size in USD Million
CAGR :
% 
 USD
891.00 Million
USD
1,927.60 Million
2025
2033
USD
891.00 Million
USD
1,927.60 Million
2025
2033
| 2026 –2033 | |
| USD 891.00 Million | |
| USD 1,927.60 Million | |
|
|
|
|
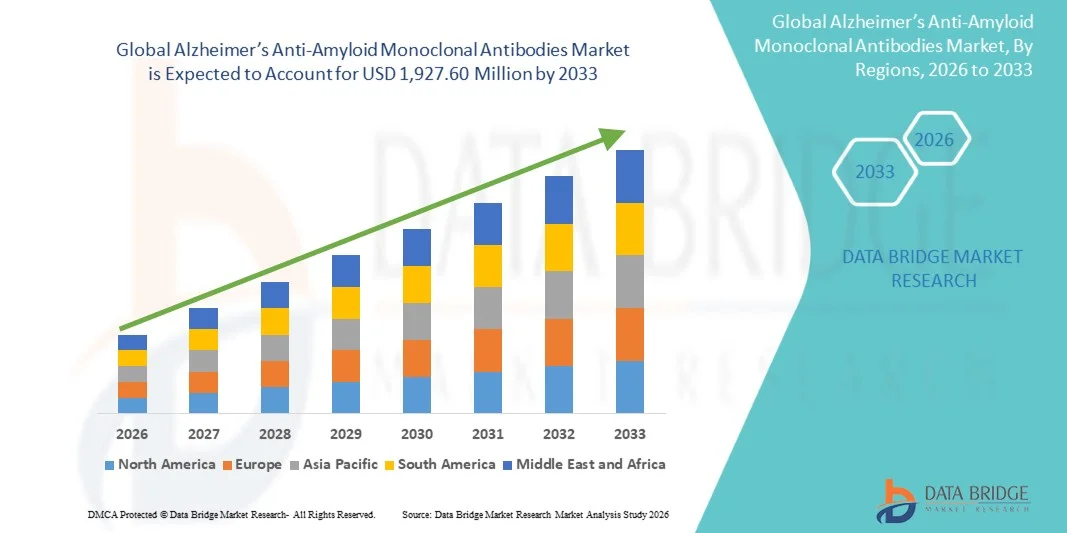
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Size
- The global Alzheimer’s Anti-Amyloid Monoclonal Antibodies market size was valued at USD 891 Million in 2025 and is expected to reach USD 1,927.60 Million by 2033, at a CAGR of 10.10% during the forecast period
- The market growth is largely driven by the rising prevalence of Alzheimer’s disease globally, increasing awareness of early diagnosis, and significant advancements in biologic drug development and disease-modifying therapies targeting amyloid pathology
- Furthermore, growing investments in neuroscience research, supportive regulatory pathways for breakthrough therapies, and increasing adoption of monoclonal antibody treatments in specialized healthcare settings are accelerating the uptake of Alzheimer’s anti-amyloid monoclonal antibodies, thereby significantly boosting the industry’s overall growth
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Analysis
- Alzheimer’s anti-amyloid monoclonal antibodies, designed to target and reduce amyloid-β plaques in the brain, are increasingly recognized as disease-modifying therapies for early-stage Alzheimer’s disease, playing a critical role in slowing cognitive decline and improving long-term patient outcomes in specialized clinical settings
- The escalating demand for these therapies is primarily driven by the rising global prevalence of Alzheimer’s disease, growing emphasis on early diagnosis and intervention, advancements in monoclonal antibody engineering, and supportive regulatory approvals for novel biologic treatments
- North America dominated the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market with the largest revenue share of approximately 44.5% in 2025, supported by a strong biotechnology and pharmaceutical ecosystem, high healthcare expenditure, extensive clinical trial activity, and early adoption of newly approved disease-modifying Alzheimer’s therapies, particularly in the U.S.
- Asia-Pacific is expected to be the fastest growing region in the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market during the forecast period, registering an estimated CAGR of around 10.2%, driven by rapidly aging populations, improving diagnostic infrastructure, increasing awareness of neurodegenerative disorders, and rising investments in advanced biologic treatments
- The Aducanumab segment dominated the largest market revenue share of approximately 42.6% in 2025, driven by its early FDA approval and adoption across hospitals and specialty neurology clinics
Report Scope and Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Segmentation
|
Attributes |
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Trends
“Increasing Focus on Disease-Modifying Therapies for Alzheimer’s”
- A significant and accelerating trend in the global Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is the growing emphasis on disease-modifying therapies aimed at slowing or halting the progression of Alzheimer’s disease rather than merely treating symptoms
- For instance, in 2024, a leading biopharmaceutical company expanded clinical trials of its next-generation anti-amyloid monoclonal antibody to target early-stage Alzheimer’s patients, highlighting the shift towards proactive disease management
- Advancements in antibody engineering, improved blood-brain barrier penetration, and personalized medicine approaches are enhancing the efficacy and safety profile of these therapies
- The increasing collaboration between academic institutions, biotechnology firms, and pharmaceutical companies is fostering innovation and accelerating the development of novel therapeutic candidates
- This trend reflects the rising demand for precision medicine and patient-centric treatment strategies in neurodegenerative disorders
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Dynamics
Driver
“Rising Alzheimer’s Prevalence and Growing Healthcare Investments”
- The market is strongly driven by the increasing prevalence of Alzheimer’s disease worldwide, particularly in aging populations, which is creating a pressing need for effective therapeutic solutions
- For instance, in 2025, North America reported over 6 million cases of Alzheimer’s, prompting significant investments in research, drug development, and patient support programs by leading pharmaceutical companies
- Healthcare systems and governments are increasingly prioritizing funding for neurodegenerative disease research, which is accelerating the commercialization and adoption of anti-amyloid monoclonal antibodies
- Moreover, expanding access to diagnostic tools such as PET imaging and cerebrospinal fluid biomarkers is facilitating earlier detection and treatment initiation, further driving market growth
- The growing awareness among clinicians and caregivers about the benefits of disease-modifying therapies is also propelling adoption across both developed and emerging markets
Restraint/Challenge
“High Treatment Costs and Regulatory Hurdles”
- Despite the potential of anti-amyloid therapies, high costs of development and treatment remain a significant barrier, particularly in low- and middle-income regions
- For instance, several healthcare providers in Europe and Asia have cited limited reimbursement coverage and affordability concerns as challenges in providing access to these therapies
- In addition, regulatory approval for Alzheimer’s disease treatments is complex due to stringent efficacy and safety requirements, variability in clinical trial outcomes, and the need for long-term post-marketing surveillance
- The requirement for specialized diagnostic tools and ongoing patient monitoring further adds to the overall treatment burden, limiting widespread adoption
- Addressing these challenges through innovative pricing models, broader insurance coverage, regulatory harmonization, and ongoing clinical validation will be essential for sustained growth in the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Scope
The market is segmented on the basis of drug type, target, and end user.
• By Drug Type
On the basis of drug type, the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is segmented into Aducanumab, Lecanemab, Donanemab, and Others. The Aducanumab segment dominated the largest market revenue share of approximately 42.6% in 2025, driven by its early FDA approval and adoption across hospitals and specialty neurology clinics. Its strong clinical trial data demonstrating amyloid beta plaque reduction, coupled with robust physician and patient awareness programs, has made it a preferred therapy. The high prevalence of Alzheimer’s disease, particularly in aging populations of North America and Europe, supports sustained market dominance. Payers and healthcare providers have increasingly incorporated Aducanumab into treatment guidelines, contributing to its significant revenue share. Market penetration in established healthcare systems, partnerships with major distributors, and ongoing post-marketing studies further reinforce its market leadership. The availability of reimbursement schemes in multiple countries enhances accessibility and adoption. Clinicians’ confidence in monitoring and managing adverse effects has also aided widespread usage. Aducanumab’s pioneering status in disease-modifying therapy has positioned it as a benchmark against which newer therapies are measured. Its established manufacturing infrastructure ensures reliable supply chains. Additionally, global awareness campaigns about early diagnosis and intervention continue to drive its clinical uptake.
The Lecanemab segment is expected to witness the fastest CAGR of 15.8% from 2026 to 2033, fueled by positive Phase 3 clinical trial outcomes demonstrating rapid cognitive improvement and amyloid reduction. Growing adoption in hospitals and specialty clinics, increased physician confidence, and ongoing regulatory approvals across multiple regions support rapid expansion. Its differentiation from competitors in terms of safety profile and dosing convenience accelerates adoption. Expanded awareness programs, patient assistance schemes, and increasing investments in neurology-focused healthcare infrastructure further contribute to growth. Market entry into emerging economies is opening new patient populations. Combination therapy research and long-term efficacy studies are also driving physician interest. Strong backing by pharmaceutical companies, targeted marketing campaigns, and payer support in key geographies enhance the growth trajectory. Healthcare providers increasingly view Lecanemab as a frontline therapy, encouraging rapid uptake. Its commercial launch in North America and Europe is gaining momentum, positioning it as a major competitor. Clinical guidelines are gradually integrating Lecanemab for early-stage Alzheimer’s, boosting revenue.
• By Target
On the basis of target, the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is segmented into Amyloid Beta Plaques, Soluble Amyloid Beta Oligomers, and Protofibrils. The Amyloid Beta Plaques segment held the largest revenue share of 47.1% in 2025, owing to established clinical evidence supporting plaque clearance as a disease-modifying mechanism. Most approved therapies, including Aducanumab and Lecanemab, primarily target plaques, driving adoption in hospitals and neurology clinics. The segment benefits from extensive clinical research, well-understood mechanisms of action, and physician familiarity. Reimbursement schemes, robust distribution networks, and patient advocacy programs further enhance market dominance. Awareness campaigns highlighting early diagnosis and intervention reinforce adoption. The segment enjoys broad geographic reach in North America, Europe, and selected Asia-Pacific markets. Continuous post-marketing studies strengthen confidence in efficacy and safety. Treatment guidelines increasingly recommend plaque-targeting agents for early Alzheimer’s stages. High physician comfort levels and structured monitoring protocols support continued preference. Access programs and institutional adoption accelerate uptake. Integration with cognitive assessment tools in clinical practice encourages consistent use. Pharmaceutical investments in scaling manufacturing capabilities ensure reliable supply.
The Soluble Amyloid Beta Oligomers segment is expected to witness the fastest CAGR of 16.3% from 2026 to 2033, driven by emerging therapies like Donanemab and investigational biologics that target soluble oligomers. Growing clinical evidence suggesting early intervention potential and superior cognitive outcomes fuels adoption. Hospitals and specialized neurology clinics are increasingly implementing oligomer-targeting therapies in early-stage Alzheimer’s. Rising R&D investments, regulatory approvals in multiple regions, and payer support contribute to accelerated growth. Ongoing combination therapy trials and biomarker-guided treatment approaches expand clinical applicability. Enhanced physician awareness and training programs on oligomer-targeting mechanisms encourage uptake. Patient education on potential disease-modifying benefits further accelerates demand. Inclusion in clinical guidelines and expert consensus statements supports adoption. Market entry into emerging economies unlocks additional growth opportunities. Positive trial outcomes regarding safety and efficacy reinforce physician confidence. Strong partnerships between pharmaceutical companies and healthcare providers facilitate distribution.
• By End User
On the basis of end user, the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is segmented into Hospitals, Specialty Neurology Clinics, Research Institutes, and Others. The Hospitals segment dominated the largest market revenue share of 44.5% in 2025, due to their extensive patient base, advanced diagnostic capabilities, and ability to administer intravenous therapies. Hospitals in North America and Europe have established infusion centers and neurology departments supporting widespread therapy administration. Integration with electronic health record systems allows structured monitoring and follow-up. Physician familiarity with disease-modifying therapies enhances adoption rates. Partnerships with pharmaceutical companies ensure reliable supply and training support. Patient access programs and insurance coverage facilitate uptake. Hospitals’ capacity to manage infusion schedules and monitor adverse events increases confidence in prescribing therapies. Multi-disciplinary care teams in hospitals enhance patient compliance and therapeutic outcomes. Geographic expansion of hospital networks continues to drive market penetration. Robust clinical infrastructure and laboratory support enable real-time biomarker assessment. Hospital protocols aligned with clinical guidelines further cement market dominance.
The Specialty Neurology Clinics segment is expected to witness the fastest CAGR of 14.9% from 2026 to 2033, propelled by increased adoption of targeted therapies, early-stage diagnosis, and individualized patient management. Clinics are expanding infusion capabilities and employing biomarker-based patient selection for therapies like Lecanemab and Donanemab. Rising awareness among neurologists and caregivers drives therapy uptake. Enhanced infrastructure, focused patient education programs, and strong collaborations with pharmaceutical companies accelerate adoption. Market penetration in urban and semi-urban regions contributes to rapid revenue growth. Specialty clinics provide personalized monitoring and dosage adjustments, enhancing patient outcomes. Payer support and reimbursement in select regions encourage adoption. Ongoing clinical studies and research collaborations improve therapeutic confidence. Technological advancements in infusion devices and patient monitoring support fast growth. Expansion of outpatient infusion services creates new revenue opportunities. Physician training programs and workshops ensure correct administration protocols.
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Regional Analysis
- North America dominated the Alzheimer’s Anti-Amyloid Monoclonal Antibodies market with the largest revenue share of approximately 44.5% in 2025, supported by a strong biotechnology and pharmaceutical ecosystem, high healthcare expenditure, extensive clinical trial activity, and early adoption of newly approved disease-modifying Alzheimer’s therapies, particularly in the U.S.
- The region benefits from robust R&D infrastructure, skilled workforce, and the presence of leading pharmaceutical and biotechnology companies. The growing prevalence of Alzheimer’s disease, coupled with increasing government and private funding for research, further strengthens market demand
- The U.S. market specifically captured a major portion, driven by rapid adoption of approved anti-amyloid therapies, advanced diagnostic infrastructure, and high patient awareness. Healthcare providers in the region prioritize innovative treatment solutions, and the integration of clinical trial outcomes into practice accelerates market growth. North America also leads in reimbursement support, facilitating patient access to high-cost monoclonal antibody therapies
U.S. Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The U.S. Alzheimer’s Anti-Amyloid Monoclonal Antibodies market captured the largest revenue share within North America in 2025, driven by the early adoption of newly approved disease-modifying therapies, advanced healthcare infrastructure, and strong clinical trial activity. The market benefits from a robust biotechnology and pharmaceutical ecosystem, high healthcare expenditure, and increasing prevalence of Alzheimer’s disease. Patients and clinicians increasingly prioritize early intervention with innovative monoclonal antibody treatments, enhancing therapy adoption. The integration of advanced diagnostics, including amyloid PET scans and CSF biomarkers, further supports early-stage detection and treatment. Reimbursement support from public and private insurers facilitates patient access to high-cost therapies. The presence of leading pharmaceutical companies with pipeline therapies underlines the U.S. market’s strategic importance.
Europe Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The Europe Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is projected to expand at a substantial CAGR throughout the forecast period, driven by stringent healthcare regulations, increasing prevalence of neurodegenerative disorders, and growing adoption of advanced biologic treatments. Countries like Germany, France, and the U.K. are witnessing steady demand due to high awareness, strong healthcare infrastructure, and government support for Alzheimer’s research. The integration of digital healthcare solutions and patient monitoring systems enhances therapy adoption. Europe is also experiencing significant growth across hospitals, specialty neurology clinics, and research institutes. Investments in clinical research, coupled with rising patient access to disease-modifying therapies, contribute to the market’s robust expansion. The market is further supported by the region’s emphasis on preventive care, early diagnosis, and treatment adherence programs.
U.K. Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The U.K. Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by an increasing number of clinical studies, adoption of disease-modifying therapies, and rising awareness of neurodegenerative disorders. Growing healthcare expenditure, coupled with robust hospital and research infrastructure, supports therapy adoption. The U.K. government’s focus on dementia care, Alzheimer’s research funding, and early diagnosis programs further accelerates market growth. Patients and clinicians increasingly prefer advanced monoclonal antibody treatments for early-stage Alzheimer’s, promoting market penetration. The U.K. also benefits from collaborations between pharmaceutical companies and research institutes, enabling quicker commercialization of newly approved therapies.
Germany Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The Germany Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is expected to expand at a considerable CAGR during the forecast period, driven by rising disease prevalence, awareness of advanced therapies, and demand for innovative biologics. Germany’s strong healthcare infrastructure, emphasis on R&D, and reimbursement policies promote therapy adoption across hospitals and specialty clinics. Patients increasingly seek disease-modifying solutions, and healthcare providers prioritize early-stage intervention. Clinical trials and collaborations between biotech firms and academic institutes further strengthen market dynamics. The focus on precision medicine and advanced diagnostic tools enhances treatment efficacy, contributing to market growth.
Asia-Pacific Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The Asia-Pacific Alzheimer’s Anti-Amyloid Monoclonal Antibodies market is expected to be the fastest growing region during the forecast period, registering an estimated CAGR of around 10.2%. Growth is driven by rapidly aging populations, improving diagnostic infrastructure, increasing awareness of neurodegenerative disorders, and rising investments in advanced biologic treatments. Countries like China, Japan, and India are witnessing expanding demand due to urbanization, increasing healthcare expenditure, and government initiatives for elderly care. The region’s adoption of newly approved therapies is also fueled by growing clinical trial activity, establishment of specialty neurology clinics, and improving access to monoclonal antibody treatments. Rising patient awareness and early diagnosis programs enhance therapy uptake across the region.
Japan Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
The Japan market is gaining momentum due to the country’s high awareness of Alzheimer’s disease, well-established healthcare infrastructure, and rapidly aging population. Growth is driven by increasing adoption of disease-modifying monoclonal antibodies, enhanced diagnostic capabilities, and integration of advanced therapies in hospitals and specialized clinics. Government support for neurodegenerative disorder research, coupled with high healthcare expenditure, further propels market expansion. The focus on early diagnosis and treatment in both residential and hospital settings enhances adoption rates. Japan also emphasizes patient education and awareness campaigns, contributing to increased acceptance of advanced monoclonal antibody therapies.
China Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Insight
China accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, rising disposable income, technological adoption, and expanding healthcare infrastructure. The country’s growing elderly population and increased awareness of Alzheimer’s disease drive demand for advanced therapies. China’s government initiatives promoting digital healthcare and early diagnosis, along with domestic pharmaceutical manufacturing capabilities, facilitate therapy accessibility. The availability of affordable monoclonal antibody options and local production of biologics support market growth. Hospitals, specialty clinics, and research institutes increasingly adopt disease-modifying therapies. Rising patient awareness programs and clinical trial activities further strengthen market uptake.
Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market Share
The Alzheimer’s Anti-Amyloid Monoclonal Antibodies industry is primarily led by well-established companies, including:
- Roche Holding AG (Switzerland)
- AC Immune SA (Switzerland)
- Novartis AG (Switzerland)
- BioArctic AB (Sweden)
- Anavex Life Sciences Corp. (U.S.)
- Cerenis Therapeutics SA (France)
- Wave Life Sciences Ltd. (U.S.)
- Vaxine Pty Ltd. (Australia)
- Denali Therapeutics Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Neuropharm Ltd. (U.K.)
- ACADIA Pharmaceuticals Inc. (U.S.)
- Fujifilm Holdings Corp. (Japan)
- Alzheon Inc. (U.S.)
- Renaissance Therapeutics (U.S.)
- BrainStorm Cell Therapeutics Inc. (U.S.)
Latest Developments in Global Alzheimer’s Anti-Amyloid Monoclonal Antibodies Market
- In January 2023, the U.S. Food and Drug Administration (FDA) granted accelerated approval to lecanemab‑irmb (brand name LEQEMBI®), a humanized anti‑amyloid monoclonal antibody for early Alzheimer’s disease, marking a significant regulatory milestone as one of the first therapies to demonstrate reduction of amyloid‑beta plaques and slowing of cognitive decline in early‑stage Alzheimer’s patients. The accelerated approval was based on positive Phase 2 data showing amyloid reduction and clinical benefit, establishing lecanemab as a pioneering disease‑modifying therapy in this space
- In July 2023, lecanemab (LEQEMBI®) received full traditional approval from the FDA for treatment of early Alzheimer’s disease following confirmatory Phase 3 results, which showed statistically significant slowing of disease progression in the Clarity AD trial. This transition from accelerated to full approval reinforced clinical confidence in lecanemab’s efficacy and supported broader commercialization efforts
- In January 2024, LEQEMBI (lecanemab) was approved in China as a treatment for mild cognitive impairment and mild Alzheimer’s dementia, making China the third major country after the U.S. and Japan to authorize this anti‑amyloid monoclonal antibody. The approval expands the geographic reach of one of the leading disease‑modifying Alzheimer’s therapies and underscores growing international acceptance of anti‑amyloid strategies
- In December 2024, Mexican health authorities approved LEQEMBI® (lecanemab) for early Alzheimer’s disease, reflecting continued global regulatory momentum for the monoclonal antibody. This approval supports expanded access to disease‑modifying treatment options in Latin America
- In July 2024, the U.S. FDA approved donanemab (brand name Kisunla), a monthly intravenous anti‑amyloid monoclonal antibody for early symptomatic Alzheimer’s disease, following positive advisory committee recommendations and clinical evidence showing that amyloid removal correlates with disease modification. Donanemab became the third anti‑amyloid mAb therapy approved in the U.S. after aducanumab and lecanemab, broadening treatment choices
- In April 2025, Eisai and Biogen announced an update on the regulatory review of lecanemab’s Marketing Authorization Application in the European Union, with the decision process advancing to the Appeal Committee stage after positive committee recommendations, indicating continued efforts to secure wider approval in key global markets
- In September 2025, the Australian Therapeutic Goods Administration approved LEQEMBI (lecanemab) for mild cognitive impairment and mild Alzheimer’s disease, based on robust Phase 3 data demonstrating significant cognitive benefits and amyloid reduction, reinforcing its global regulatory expansion
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.