Global Alzheimers Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
5.99 Billion
USD
11.39 Billion
2024
2032
USD
5.99 Billion
USD
11.39 Billion
2024
2032
| 2025 –2032 | |
| USD 5.99 Billion | |
| USD 11.39 Billion | |
|
|
|
|
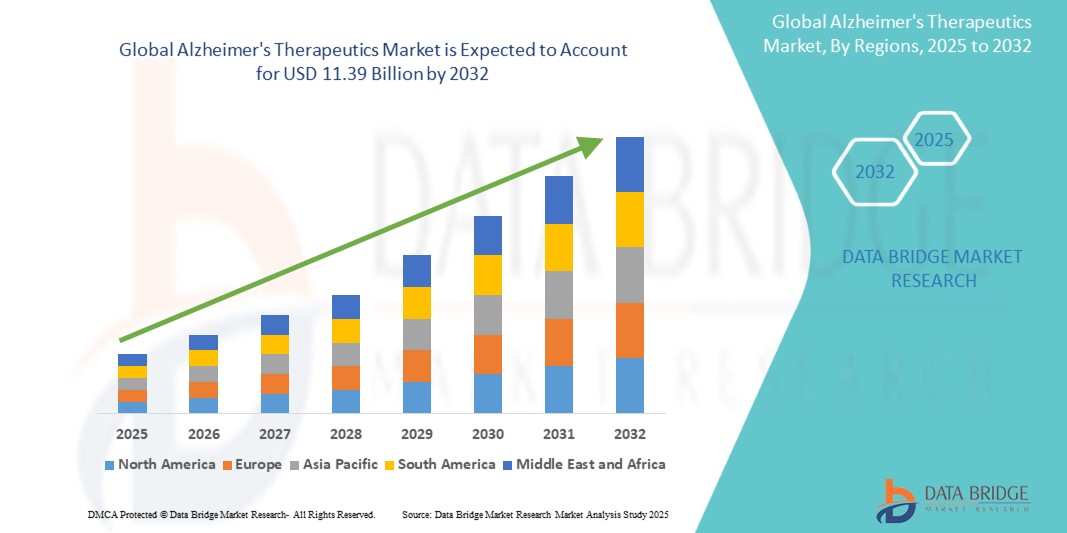
Alzheimer's Therapeutics Market Size
- The global Alzheimer’s therapeutics market was valued at USD 5.99 billion in 2024 and is expected to reach USD 11.39 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 8.36%, primarily driven by the increasing prevalence of Alzheimer's disease
- This growth is driven by factors such as the aging population, rising prevalence of Alzheimer’s disease and advancements in drug development and FDA approvals
Alzheimer's Therapeutics Market Analysis
- Alzheimer’s therapeutics refer to treatments developed to manage the symptoms of Alzheimer’s disease, slow its progression, and improve the quality of life for patients. These include symptomatic treatments, such as cholinesterase inhibitors and glutamate regulators, as well as disease-modifying therapies that aim to alter the course of the disease
- The market for Alzheimer’s therapeutics has been expanding rapidly, driven by the growing global prevalence of the disease, particularly as the population ages
- The demand for Alzheimer’s therapeutics is significantly influenced by the rising number of individuals diagnosed with the disease, especially in regions with aging populations such as North America and Europe. As these regions see an increasing number of Alzheimer's patients, there is heightened focus on developing effective treatments. Advancements in research and the approval of new drugs also contribute to market growth
- For instance, In North America, the approval of Aduhelm by the FDA in June 2021 marked a significant milestone in Alzheimer's treatment. This drug, developed by Biogen, targets amyloid plaques, a hallmark of Alzheimer's, offering a new treatment option and driving the demand for disease-modifying therapies
- Overall, the Alzheimer's therapeutics market is expected to grow significantly as demand for innovative treatments rises alongside the aging global population
Report Scope and Alzheimer's Therapeutics Market Segmentation
|
Attributes |
Alzheimer's Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Alzheimer's Therapeutics Market Trends
“Increasing Focus on Disease-Modifying Therapies”
- One prominent trend in the global Alzheimer's therapeutics market is the increasing focus on disease-modifying therapies (DMTs) that aim to slow or halt the progression of Alzheimer’s disease
- These therapies target the underlying biological processes of the disease, such as amyloid plaques and tau tangles, offering hope for more effective treatments beyond symptomatic relief
- For instance, Aduhelm, developed by Biogen, is one of the first FDA-approved DMTs that targets amyloid beta plaques, a hallmark of Alzheimer’s, offering potential disease-modifying effects and sparking interest in other similar treatments
- In addition, advancements in biomarker development are enabling earlier diagnosis and more personalized treatment approaches, allowing therapies to be administered at the most beneficial stages of the disease
- This trend is reshaping the Alzheimer’s treatment landscape, with increased investment in research and development of innovative drugs aimed at slowing or preventing disease progression, which is fueling the market's growth and demand for new therapeutic options
Alzheimer's Therapeutics Market Dynamics
Driver
“Rising Prevalence of Alzheimer’s Disease”
- The rising prevalence of Alzheimer’s disease is significantly contributing to the increased demand for Alzheimer’s therapeutics
- As the global population ages, the incidence of Alzheimer’s continues to rise, with older adults being more susceptible to cognitive decline and dementia-related disorders
- Alzheimer’s disease is one of the most common causes of dementia, leading to a growing need for effective treatments that can manage symptoms and potentially slow disease progression
- Ongoing advancements in Alzheimer’s research, including the development of disease-modifying therapies (DMTs), highlight the need for innovative drug options that address the root causes of Alzheimer’s, such as amyloid plaques and tau tangles
- As more individuals are diagnosed with Alzheimer’s, the demand for therapeutics continues to rise, contributing to increased market growth and an emphasis on research to develop novel treatment solutions
For instance,
- In June 2021, the U.S. FDA approved Aduhelm (aducanumab), a disease-modifying therapy for Alzheimer’s. This marked a significant step forward in Alzheimer's treatment and demonstrated the increasing demand for innovative Alzheimer’s therapeutics, addressing the growing global prevalence of the disease
- In October 2022, the World Health Organization (WHO) reported that the global number of people living with dementia is projected to increase from 57 million in 2019 to 152 million by 2050, highlighting the increasing global burden of Alzheimer's disease and fueling the demand for more effective therapeutics
- As a result of the rising prevalence of Alzheimer’s disease, there is a significant increase in the demand for Alzheimer’s therapeutics
Opportunity
“Development Of Biomarker-Based Diagnostics for Alzheimer’s Disease”
- The development of biomarker-based diagnostics for Alzheimer’s disease presents a significant opportunity in the Alzheimer’s therapeutics market
- Biomarkers can provide early and accurate detection of Alzheimer’s, enabling clinicians to identify patients at higher risk and begin treatment earlier, potentially slowing disease progression
- This opportunity is driving the development of diagnostic tools and therapies that focus on detecting specific biomarkers related to Alzheimer’s, which could lead to more personalized and effective treatment options
For instance,
- In December 2023, according to an article published in the Journal of Alzheimer's Disease, the discovery of novel biomarkers such as tau and amyloid proteins is helping researchers create tests that could detect Alzheimer’s at earlier stages, offering the potential for intervention before significant cognitive decline occurs
- In March 2024, an article published in Nature Reviews Neurology highlighted the role of blood-based biomarkers, which could offer a less invasive and cost-effective means to diagnose Alzheimer’s, significantly expanding accessibility to early diagnosis and treatment
- The integration of biomarkers in both diagnostic and therapeutic strategies can improve the accuracy of diagnosis, enable earlier intervention, and ultimately enhance patient outcomes, contributing to the growth of the Alzheimer’s therapeutics market
Restraint/Challenge
“High Cost of Alzheimer’s Therapeutics”
- The high cost of Alzheimer’s therapeutics poses a significant challenge for the market, particularly affecting the affordability and accessibility of treatments, especially in low-income and developing regions
- These therapies, especially newer disease-modifying treatments, can cost tens of thousands of dollars annually, making them out of reach for many patients and healthcare systems
- This financial barrier can prevent wider adoption of these therapies, leading to limited access for those who could benefit the most, especially in regions with less developed healthcare infrastructures
For instance,
- In May 2024, according to an article published by the National Institute on Aging, the high cost of medications such as Aduhelm has raised concerns about their affordability, with some patients and healthcare providers unable to cover the costs, limiting the broader accessibility of this treatment
- Consequently, such financial constraints can result in disparities in the quality of care, hindering the growth and adoption of Alzheimer’s therapeutics in certain regions and populations
Alzheimer's Therapeutics Market Scope
The market is segmented on the basis of product type, route of administration, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Route of Administration |
|
|
By End User |
|
Alzheimer's Therapeutics Market Regional Analysis
“North America is the Dominant Region in the Alzheimer's Therapeutics Market”
- North America dominates the Alzheimer’s therapeutics market, driven by advanced healthcare infrastructure, high adoption of innovative treatments, and a strong presence of key pharmaceutical companies
- The U.S. holds a significant share due to the increased demand for Alzheimer’s treatments, rising prevalence of Alzheimer’s disease, and continuous advancements in therapeutic approaches
- The availability of well-established healthcare reimbursement policies and growing investments in research & development by leading pharmaceutical companies further strengthen the market
- In addition, the rising number of people diagnosed with Alzheimer’s disease, particularly among the aging population, coupled with increasing awareness and early diagnosis, is fueling market expansion across the region
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific is expected to witness the highest growth rate in the Alzheimer's therapeutics market
- This growth is driven by the rapidly aging population in countries such as China, Japan, and India, as well as increased awareness of Alzheimer’s disease and advancements in healthcare infrastructure. The growing burden of Alzheimer’s in these countries, coupled with rising government investments in healthcare and a stronger focus on neurodegenerative disease treatments, contributes to the region's rapid market expansion
- China is expected to witness the highest growth rate in the Alzheimer's therapeutics market. The rapid aging population, growing awareness of Alzheimer’s disease, and significant improvements in healthcare infrastructure are driving the demand for Alzheimer’s treatments. Moreover, increasing government initiatives to combat age-related diseases and a rising number of Alzheimer's diagnoses contribute to China’s anticipated market growth
Alzheimer's Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- ADvantage Therapeutics (U.S.)
- Alzheon, Inc. (U.S.)
- Annovis Bio, Inc. (U.S.)
- Alpha Cognition (Canada)
- Amneal Pharmaceuticals LLC (U.S.)
- AbbVie Inc. (U.S.)
- Aurobindo Pharma Limited (India)
- Biogen (U.S.)
- Cassava Sciences, Inc. (U.S.)
- Dr. Reddy’s Laboratories Ltd. (India)
- Eisai Co., Ltd. (Japan)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- Lilly (U.S.)
- Novartis AG (Switzerland)
- Sanofi (France)
- TauRx Pharmaceuticals Ltd. (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Viatris Inc. (U.S.)
Latest Developments in Global Alzheimer's Therapeutics Market
- In December 2024, Eisai Co., Ltd. and Biogen Inc. announced that Mexico's Federal Commission for the Protection Against Sanitary Risk (COFEPRIS) has approved LEQEMBI (lecanemab), a humanized anti-soluble aggregated amyloid-beta (Aβ) monoclonal antibody, for the treatment of early Alzheimer’s disease (AD)
- In July 2024, the U.S. Food and Drug Administration (FDA) granted approval for Kisunla (donanemab-azbt, 350 mg/20 mL once-monthly injection for IV infusion), an Alzheimer’s treatment by Eli Lilly and Company. This approval is for adults with early symptomatic Alzheimer's disease (AD), including those with mild cognitive impairment (MCI) and individuals in the mild dementia stage of AD, who have confirmed amyloid pathology
- In July 2024, Alpha Cognition announced that the U.S. Food and Drug Administration (FDA) has approved ZUNVEYL (benzgalantamine), formerly known as ALPHA-1062, for the treatment of mild-to-moderate Alzheimer's disease. ZUNVEYL features a dual mechanism of action designed to prevent drug absorption in the gastrointestinal tract, potentially addressing tolerability issues associated with leading Alzheimer's medications, while offering the efficacy and long-term benefits of galantamine
- In January 2024, Biogen Inc. revealed its plans to refocus its resources on Alzheimer’s disease (AD), a key therapeutic area projected to drive both short-term and long-term growth. The company will continue to progress LEQEMBI (lecanemab-irmb), the first anti-amyloid beta treatment to receive FDA traditional approval in the U.S., and will expedite the development of potential new treatment options, such as its ASO targeting tau (BIIB080) and an oral small molecule inhibitor for tau aggregation (BIIB113)
- In May 2023, Eli Lilly and Company announced positive results from the TRAILBLAZER-ALZ 2 Phase 3 study, demonstrating that donanemab significantly slowed cognitive and functional decline in individuals with early symptomatic Alzheimer's disease. Donanemab successfully met the primary endpoint of change from baseline to 18 months on the integrated Alzheimer's Disease Rating Scale (iADRS)
- In January 2023, the U.S. Food and Drug Administration granted Accelerated Approval for Leqembi (lecanemab-irmb) as a treatment for Alzheimer's disease. Leqembi is the second medication in a new class designed to target the underlying pathophysiology of Alzheimer's disease
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.