Global Anaplastic Lymphoma Kinase Metastatic Non Small Cell Lung Cancer Market
Market Size in USD Billion
CAGR :
% 
 USD
2.14 Billion
USD
3.65 Billion
2024
2032
USD
2.14 Billion
USD
3.65 Billion
2024
2032
| 2025 –2032 | |
| USD 2.14 Billion | |
| USD 3.65 Billion | |
|
|
|
|
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Size
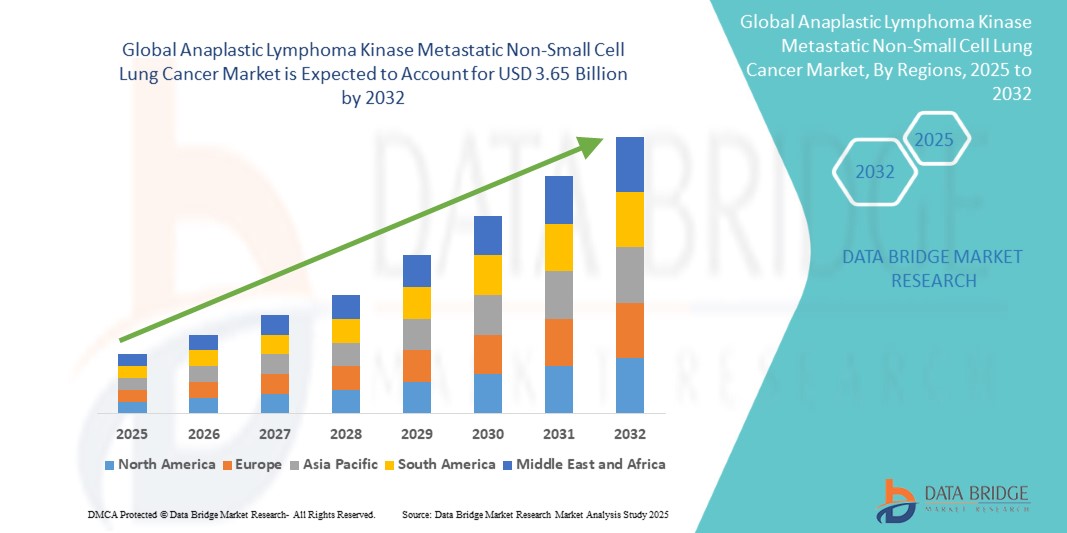
- The global anaplastic lymphoma kinase metastatic non-small cell lung cancer market was valued at USD 2.14 billion in 2024 and is expected to reach USD 3.65 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 6.92%, primarily driven by the increasing targeted therapy adoption
- This growth is driven by factors such as the aging population, increasing adoption of targeted therapies for ALK-positive metastatic non-small cell lung cancer and growing awareness and early diagnosis
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Analysis
- Anaplastic Lymphoma Kinase (ALK) Metastatic Non-Small Cell Lung Cancer (NSCLC) is a subtype of lung cancer characterized by mutations in the ALK gene, leading to abnormal cell growth and. ALK-positive NSCLC accounts for approximately 3-5% of all NSCLC cases. This mutation drives the cancer's progression and makes it amenable to targeted therapies that specifically inhibit the abnormal ALK protein, offering more effective and less toxic treatment compared to traditional chemotherapy
- The market for ALK metastatic NSCLC is projected to grow at a CAGR of 6.92%. This growth is primarily fueled by advancements in targeted therapies, with drugs such as crizotinib, alectinib, brigatinib, and lorlatinib showing strong efficacy in treating ALK-positive patients. These treatments block the activity of the mutated ALK protein, significantly improving survival rates and quality of life for patients
- For instance, alectinib (Alecensa), an ALK inhibitor, has demonstrated superior efficacy compared to crizotinib in treating ALK-positive NSCLC, leading to its widespread adoption in clinical practice
- Moreover, ongoing clinical trials and the development of next-generation therapies are expected to further propel market growth. Enhanced awareness, early diagnosis, and improved molecular profiling technologies are also contributing to the increasing number of patients receiving tailored treatments
Report Scope and Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Segmentation
|
Attributes |
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Trends
“Growing Adoption of Next-Generation ALK Inhibitors”
- One prominent trend in the Anaplastic Lymphoma Kinase (ALK) metastatic non-small cell lung cancer (NSCLC) market is the growing adoption of next-generation ALK inhibitors
- These advanced therapies, such as lorlatinib and brigatinib, are designed to overcome resistance to earlier ALK inhibitors, providing more effective treatment options for patients with advanced-stage ALK-positive NSCLC
- For instance, lorlatinib has demonstrated superior efficacy in patients whose cancer has developed resistance to crizotinib, offering improved progression-free survival and better control of the disease
- These next-generation ALK inhibitors not only enhance treatment outcomes but also have the potential to target a broader range of ALK mutations, offering a more personalized treatment approach for patients
- This trend is reshaping the treatment landscape for ALK-positive NSCLC, driving market growth and increasing the demand for these advanced therapies
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Dynamics
Driver
“Increasing adoption of targeted therapies for ALK-positive metastatic non-small cell lung cancer”
- The increasing adoption of targeted therapies for ALK-positive metastatic non-small cell lung cancer is driving the growth of the market
- With advancements in the understanding of molecular profiling, there is a growing emphasis on precision medicine, allowing healthcare providers to offer tailored treatments for ALK-positive patients
- Targeted therapies such as ALK inhibitors (such as crizotinib, alectinib, and lorlatinib) have significantly improved patient outcomes by offering more effective treatments with fewer side effects compared to traditional chemotherapy
- The growing recognition of the benefits of these targeted treatments is driving demand for ALK inhibitors, further accelerating market growth
For instance,
- In November 2020, the FDA approved lorlatinib for the treatment of ALK-positive metastatic NSCLC after resistance to earlier ALK inhibitors. This approval highlights the increasing reliance on targeted therapies and precision medicine in treating ALK-positive NSCLC, driving market growth
- In March 2021, the FDA granted approval for alectinib as a first-line treatment for ALK-positive metastatic NSCLC in adult patients. This approval further underscores the growing emphasis on targeted therapies, providing patients with more effective and less toxic options compared to traditional chemotherapy
- Ongoing clinical trials continue to explore new drug candidates for ALK-positive NSCLC, expanding treatment options and further driving market demand for these advanced therapies
Opportunity
“Increasing Demand for Personalized Medicine”
- Increasing demand for personalized medicine offers a significant opportunity in the ALK-positive metastatic NSCLC market, as advancements in genetic profiling and biomarker testing allow for more tailored treatment options
- By identifying specific ALK mutations in patients, targeted therapies can be more effectively selected, leading to improved treatment outcomes and minimized side effects
- In addition, personalized medicine enables the development of novel ALK inhibitors and combination therapies, further expanding the market and providing more effective treatment options for patients with resistant or advanced-stage disease
For instance,
- In June 2020, the FDA approved the use of Comprehensive Genomic Profiling (CGP) to detect ALK mutations in patients with metastatic NSCLC. This approval highlights the growing use of personalized medicine in the treatment of ALK-positive NSCLC, allowing for more targeted and effective therapy options based on individual genetic profiles
- In August 2021, the results of a clinical trial published in JCO Precision Oncology demonstrated that combination therapy using ALK inhibitors with other targeted treatments significantly improved progression-free survival in patients with resistant ALK mutations. This reinforces the value of personalized treatment strategies and the continued development of tailored therapies for ALK-positive metastatic NSCLC
- The increasing demand for personalized medicine in the ALK-positive metastatic NSCLC market can lead to significantly improved patient outcomes, enhanced treatment efficacy, and reduced side effects. By leveraging genetic profiling and biomarker testing, healthcare providers can select the most effective targeted therapies, ensuring better disease control and improved quality of life for patients. In addition, personalized medicine helps in identifying patients who may benefit from combination therapies, further enhancing treatment success and reducing resistance to drugs
Restraint/Challenge
“High Cost of ALK Inhibitors”
- The high cost of ALK inhibitors for metastatic NSCLC presents a significant restraint for the market, particularly limiting access to these therapies in lower-income regions and among underinsured populations
- These targeted therapies, essential for treating ALK-positive NSCLC, can often cost tens of thousands of dollars per year, creating a financial barrier for both patients and healthcare systems
- This substantial cost can hinder the widespread adoption of these treatments, particularly in developing countries or smaller healthcare settings with budget constraints, leading to reliance on less effective or outdated treatment options
For instance,
- In March 2023, according to a report published by the American Lung Association, the high cost of ALK inhibitors, such as crizotinib and alectinib, continues to be a major obstacle for many patients in accessing the latest treatment options. This cost burden affects both healthcare providers and patients, ultimately limiting the reach of these breakthrough therapies and hindering market growth
- Consequently, the financial barrier imposed by the high cost of these drugs may delay treatment initiation or force healthcare providers to choose less optimal therapies, ultimately affecting patient outcomes
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Scope
The market is segmented on the basis of type and applications covered.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Applications Covered |
|
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Regional Analysis
“North America is the Dominant Region in the Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market”
- North America dominates the Anaplastic Lymphoma Kinase (ALK) metastatic non-small cell lung cancer (NSCLC) market, driven by advanced healthcare infrastructure, high adoption of targeted therapies, and a strong presence of leading pharmaceutical companies
- The U.S. holds a significant share due to the increasing prevalence of ALK-positive metastatic NSCLC, rising demand for targeted therapies, and continuous advancements in treatment options, particularly ALK inhibitors such as crizotinib, alectinib, and lorlatinib
- The availability of well-established reimbursement policies and ongoing investments in research & development by key pharmaceutical companies, including Novartis, Pfizer, and Roche, further strengthen the market in North America
- In addition, the growing number of ALK-positive NSCLC diagnoses, improved genetic profiling technologies, and the rapid adoption of precision medicine are fueling market expansion across the region. The increasing focus on personalized treatments and early diagnosis through molecular testing also contributes to the market's growth
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific is expected to witness the highest growth rate in the Anaplastic Lymphoma Kinase (ALK) metastatic non-small cell lung cancer (NSCLC) market, driven by rapid improvements in healthcare infrastructure, increasing awareness about lung cancer, and rising adoption of targeted therapies
- Countries such as China, India, and Japan are emerging as key markets due to the rising incidence of ALK-positive metastatic NSCLC and growing demand for precision medicine and targeted treatments
- China is projected to register the highest growth rate in the Anaplastic Lymphoma Kinase (ALK) metastatic non-small cell lung cancer (NSCLC) market. This growth is driven by several factors, including the rising incidence of lung cancer, particularly ALK-positive NSCLC, the increasing adoption of targeted therapies, and the expansion of healthcare infrastructure
Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- AstraZeneca (U.K.)
- Akeso Biopharma Co., Ltd. (China)
- Amgen Inc. (U.S.)
- Betta Pharmaceuticals Co., Ltd. (China)
- Blueprint Medicines Corporation (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland) Bristol-Myers Squibb Company (U.S.)
- Eisai Co., Ltd. (Japan)
- GSK plc. (UK)
- Lilly (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Xcovery (U.S.)
Latest Developments in Global Anaplastic Lymphoma Kinase Metastatic Non-Small Cell Lung Cancer Market
- In December 2024, the Food and Drug Administration (FDA) approved ensartinib (Ensacove, Xcovery Holdings, Inc.) for the treatment of adult patients with ALK-positive locally advanced or metastatic non-small cell lung cancer (NSCLC) who have not been previously treated with an ALK inhibitor
- In May 2024, Pfizer Inc. announced the long-term follow-up results from the Phase 3 CROWN trial, which evaluated LORBRENA (lorlatinib, a third-generation ALK inhibitor, marketed in Europe as LORVIQUA) compared to XALKORI (crizotinib) in patients with previously untreated ALK-positive advanced non-small cell lung cancer (NSCLC)
- In April 2024, the Food and Drug Administration approved alectinib (Alecensa, Genentech, Inc.) for adjuvant treatment following tumour resection in patients with ALK-positive non-small cell lung cancer (NSCLC), as identified through an FDA-approved test
- In September 2023, Merck and Eisai shared updates on two Phase 3 trials, LEAP-006 and LEAP-008, assessing the combination of KEYTRUDA, Merck's anti-PD-1 therapy, and LENVIMA, the orally available multi-receptor tyrosine kinase inhibitor developed by Eisai, in patients with specific types of metastatic non-small cell lung cancer
- In March 2021, the U.S. Food and Drug Administration (FDA) approved Pfizer Inc.’s supplemental New Drug Application (sNDA) for LORBRENA (lorlatinib), broadening its indication to include first-line treatment for patients with ALK-positive non-small cell lung cancer (NSCLC). LORBRENA is now approved for adults with metastatic NSCLC whose tumors are ALK-positive, as identified by an FDA-approved test
- In January 2021, Takeda Pharmaceutical Company Limited announced that it received approval from the Japanese Ministry of Health, Labour and Welfare to manufacture and market ALUNBRIG Tablets 30 mg and 90 mg (brigatinib, development code: AP26113) as a first- and second-line treatment for patients with unresectable, advanced, or recurrent ALK fusion gene-positive non-small cell lung cancer (ALK+ NSCLC)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.