Global Aniridia Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
43.25 Billion
USD
59.63 Billion
2024
2032
USD
43.25 Billion
USD
59.63 Billion
2024
2032
| 2025 –2032 | |
| USD 43.25 Billion | |
| USD 59.63 Billion | |
|
|
|
|
Aniridia Treatment Market Size
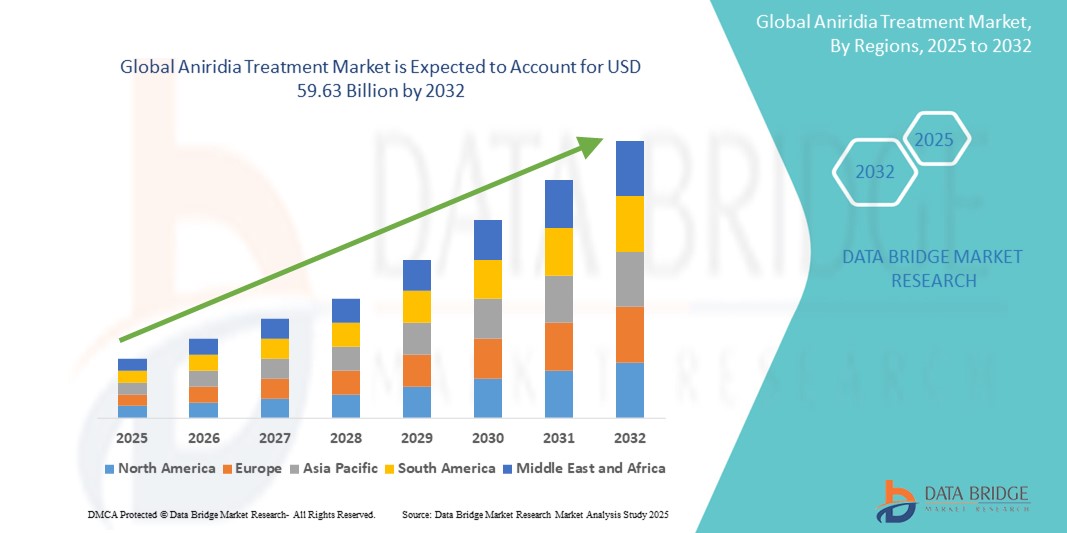
- The global aniridia treatment market size was valued at USD 43.25 billion in 2024 and is expected to reach USD 59.63 billion by 2032, at a CAGR of 4.10% during the forecast period
- The market growth is largely fueled by the rising awareness and early diagnosis of rare ocular conditions such as aniridia, along with growing advancements in genetic testing and personalized medicine. Increasing healthcare investments and greater access to specialized eye care are enabling earlier intervention and expanding treatment opportunities globally
- Furthermore, the growing demand for targeted and regenerative therapies for ocular disorders is driving innovation in the aniridia treatment market. These converging factors are accelerating the uptake of aniridia treatment solutions—including limbal stem cell therapy, prosthetic iris implants, and novel pharmacological approaches—thereby significantly boosting the industry’s growth
Aniridia Treatment Market Analysis
- Aniridia treatment refers to the therapeutic management of congenital or acquired absence of the iris, a rare genetic condition often linked to PAX6 gene mutations and associated with complications such as glaucoma, cataracts, and corneal opacification
- The growing prevalence of genetic eye disorders, increasing awareness among ophthalmologists, and advancements in gene therapy, regenerative medicine, and corneal surface reconstruction are key drivers propelling the growth of the Aniridia Treatment market.
- North America dominated the aniridia treatment market with the largest revenue share of 38.4% in 2024, attributed to early diagnostic capabilities, advanced healthcare infrastructure, and active clinical research initiatives, particularly in the U.S. The region has seen strong uptake of investigational therapies and specialized surgical procedures for vision preservation in pediatric and adult patients
- Asia-Pacific is projected to grow at the fastest CAGR of 8.6% during the forecast period (2025–2032) due to rising patient awareness, increased ophthalmology care access, and a surge in demand for innovative therapies in countries such as Japan, China, and India
- The topical treatment segment led the aniridia treatment market with a share of 41.2% in 2024, driven by the widespread use of lubricating eye drops, anti-inflammatory agents, and medications for managing related symptoms like dry eye and glaucoma
- The minimally invasive surgery segment led the market with the largest share of 51.4% in 2024, supported by growing patient preference for procedures with less pain and quicker recovery
Report Scope and Aniridia Treatment Market Segmentation
|
Attributes |
Aniridia Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Aniridia Treatment Market Trends
“Growing Need Due to Rising Disease Burden and Advancements in Genetic Therapies”
- The increasing incidence of congenital eye disorders such as aniridia, driven by genetic mutations such as PAX6 gene anomalies, is a significant driver of the aniridia treatment market. The rising demand for early diagnosis and targeted treatments is creating strong momentum for therapeutic innovation
- For instance, in 2024, several biotech firms initiated clinical trials focusing on stem-cell-based and gene therapies targeting rare ocular disorders, including Aniridia. These pipeline developments are expected to fuel growth in the coming years
- As awareness grows among patients, families, and clinicians, the demand for personalized ophthalmology treatments is expanding. Newer treatments aim not only to manage symptoms like photophobia and keratopathy but also to modify disease progression
- Furthermore, regulatory incentives like orphan drug designation and accelerated approval pathways are encouraging pharmaceutical companies to invest in R&D for Aniridia. This has led to the emergence of novel biologics, regenerative therapies, and pharmacologic agents specifically designed for rare ocular conditions
- The rising emphasis on multidisciplinary care approaches, combining ophthalmologists, geneticists, and pediatricians, is enhancing patient outcomes and expanding the adoption of advanced Aniridia therapies across hospitals and specialty clinics
Aniridia Treatment Market Dynamics
Driver
“Increasing Demand for Advanced and Targeted Aniridia Treatments”
- A significant and accelerating trend in the global aniridia treatment market is the rising emphasis on personalized and targeted therapeutic options tailored to address the underlying genetic and ocular complications of the condition. This includes advancements in both pharmacological treatments and surgical interventions designed to preserve and enhance visual function in affected individuals
- For instance, topical formulations with cyclosporine or corticosteroids are being increasingly prescribed to manage associated conditions such as dry eye and inflammation. These treatments help mitigate discomfort and delay the progression of corneal damage in patients with Aniridia
- The development of novel regenerative and gene therapy-based approaches is gaining momentum, with researchers exploring ways to address PAX6 gene mutations—the primary cause of Aniridia. Ongoing clinical trials are evaluating the potential of stem cell therapy and targeted molecular treatments to restore or preserve visual acuity
- Pharmaceutical innovations also include preservative-free eye drops, autologous serum eye drops, and anti-VEGF agents aimed at reducing the progression of associated complications such as glaucoma and aniridia-associated keratopathy (AAK), which significantly impact quality of life
- Surgical interventions like limbal stem cell transplants and customized keratoprosthesis are being adopted in severe cases where corneal clarity is compromised. Such approaches are supported by increasing healthcare investments and the rise of specialized ophthalmology centers across major markets
- The demand for integrated, long-term treatment protocols that combine pharmacologic, surgical, and rehabilitative strategies is growing rapidly across both pediatric and adult populations. This is driven by increasing awareness, better diagnostic tools, and the growing emphasis on improving patient outcomes and vision-related quality of life
Restraint/Challenge
“High Cost and Limited Awareness in Emerging Markets”
- The high cost of gene therapies and biologics remains a major barrier to access for many patients, especially in developing regions. Advanced treatments like recombinant growth factors or stem cell transplantation require significant healthcare infrastructure and specialized personnel, further increasing overall treatment expenses
- Moreover, limited public and clinical awareness regarding Aniridia, particularly in underdeveloped countries, leads to delayed diagnosis and suboptimal management. This lack of education among primary care providers hinders early intervention, which is critical in preserving vision
- For instance, reports suggest that many patients in low-income regions remain undiagnosed or are misdiagnosed with other vision disorders, delaying access to appropriate care
- In addition, reimbursement challenges and lack of coverage for rare disease treatments in national healthcare systems act as a deterrent for patients and providers to adopt cutting-edge Aniridia therapies
- Overcoming these challenges will require a combination of policy-level interventions, affordability programs, clinician training, and greater integration of genetic testing and early screening programs in public health systems
Aniridia Treatment Market Scope
The market is segmented on the basis of product, disease, technology, application, and end-users.
- By Product
On the basis of product, the aniridia treatment market is segmented into instruments, dialysis devices, endoscopes, laser and lithotripsy devices, endovision and imaging devices, robotic systems, insufflators, endoscopy fluid management systems, urodynamic systems, and consumables and accessories. The consumables and accessories segment dominated the market with the largest revenue share of 36.5% in 2024, due to high usage rates and the necessity for frequent replacement in clinical settings.
The robotic systems segment is expected to grow at the fastest CAGR of 9.6% from 2025 to 2032, driven by increasing adoption of precision surgical systems across urology and ophthalmology practices.
- By Disease
On the basis of disease, the aniridia treatment market is segmented into kidney diseases, urological cancer and benign prostatic hyperplasia (BPH), pelvic organ prolapse, and other diseases. The Urological Cancer and BPH segment captured the largest market share of 42.3% in 2024, owing to rising incidence and awareness of prostate health among aging populations.
The pelvic organ prolapse segment is projected to witness a fastest CAGR of 8.1% during 2025–2032, driven by increasing demand for minimally invasive treatment solutions among women.
- By Technology
On the basis of technology, the aniridia treatment market is segmented into minimally invasive surgery, robotic surgery, and others. Minimally invasive surgery led the market with a share of 51.4% in 2024, supported by growing patient preference for procedures with less pain and quicker recovery.
Robotic surgery is anticipated to expand at the fastest CAGR of 10.4% from 2025 to 2032, backed by rising installations of robotic platforms in tertiary care hospitals and specialty centers.
- By Application
On the basis of application, the aniridia treatment market is segmented into benign prostatic hyperplasia, prostate cancer, urinary stones, urinary incontinence, and others. The prostate cancer segment held the dominant share of 38.7% in 2024, driven by increasing use of laser therapy, radiotherapy, and robotic-assisted surgical treatments.
The urinary incontinence segment is forecast to grow at a fastest CAGR of 8.9% through 2032, supported by a rising elderly population and advancements in diagnostic tools and devices.
- By End-Users
On the basis of end-users, the aniridia treatment market is segmented into hospitals and clinics, dialysis centres, ambulatory services, and others. Hospitals and Clinics accounted for the largest revenue share of 58.1% in 2024, due to their comprehensive care capabilities, better infrastructure, and higher patient inflow.
Ambulatory services are projected to record the fastest CAGR of 9.1% from 2025 to 2032, reflecting the shift toward outpatient care and minimally invasive procedures.
Aniridia Treatment Market Regional Analysis
- North America dominated the aniridia treatment market with the largest revenue share of 38.4% in 2024, driven by increasing awareness of rare genetic eye disorders, a strong presence of advanced healthcare infrastructure, and robust R&D activities focused on ocular disease treatments. The presence of key pharmaceutical companies and a favorable regulatory environment for orphan drug development further support regional growth
- Patients and healthcare providers in the region are highly receptive to novel treatments, including gene therapies and stem-cell-based options, especially for pediatric aniridia. Furthermore, funding from government and private organizations for rare disease research is accelerating the development of targeted therapies for aniridia
- This widespread development and adoption of advanced therapeutic options are further supported by high healthcare expenditure, rapid diagnostics, and well-structured insurance coverage, reinforcing North America’s dominance in the global aniridia treatment market
U.S. Aniridia Treatment Market Insight
The U.S. aniridia treatment market captured the largest revenue share of 86.1% in 2024 within North America, owing to the country's leadership in rare disease research, access to advanced treatment centers, and early approvals for gene therapy products. Organizations such as the NIH and the FDA’s Orphan Drug Program continue to drive innovation. In addition, rising patient awareness and increased enrollment in clinical trials are contributing to a growing treatment pool and overall market expansion.
Europe Aniridia Treatment Market Insight
The Europe aniridia treatment market is projected to expand at a substantial CAGR from 2025 to 2032, primarily fueled by increasing prevalence of genetic disorders, strong support for rare disease registries, and improved diagnostics. Countries such as Germany, France, and the U.K. are leading the way in funding programs that encourage research in congenital ocular conditions. Public health initiatives and rising demand for early intervention therapies are further promoting market growth.
U.K. Aniridia Treatment Market Insight
The U.K. aniridia treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by NHS funding for orphan drugs, rising genetic screening awareness, and a push toward patient-centered personalized treatments. In addition, collaborative research between academic institutions and biotech firms is driving innovation in non-invasive and regenerative therapies.
Germany Aniridia Treatment Market Insight
The Germany aniridia treatment market is expected to expand at a considerable CAGR during the forecast period, attributed to its well-established clinical trial infrastructure, growing focus on pediatric ophthalmology, and the presence of global pharmaceutical firms investing in eye-related gene therapies. Rising demand for targeted therapies and advanced diagnostics in Germany supports the increasing adoption of treatment options for rare conditions like aniridia.
Asia-Pacific Aniridia Treatment Market Insight
The Asia-Pacific aniridia treatment market is poised to grow at the fastest CAGR of 8.6% during the forecast period of 2025 to 2032, driven by improving healthcare access, rapid urbanization, and growing government investments in rare disease awareness and screening programs. Countries like China, Japan, and India are experiencing a surge in genetic testing and early diagnosis initiatives, which are critical for effective aniridia management.
Japan Aniridia Treatment Market Insight
The Japan aniridia treatment market is gaining momentum, propelled by advanced healthcare technologies, rising awareness of pediatric ocular diseases, and a growing interest in stem cell research. The Japanese government’s support for rare disease treatment and early detection is fostering innovation in therapies, particularly those focused on ocular surface regeneration and corneal repair.
China Aniridia Treatment Market Insight
The China aniridia treatment market accounted for the largest revenue share in the Asia-Pacific region in 2024, driven by a rapidly growing middle class, increasing availability of diagnostic services, and expanding pharmaceutical manufacturing capabilities. Local research institutions are actively participating in global clinical trials, and domestic companies are beginning to enter the rare disease therapeutics space, particularly through biosimilars and gene therapy development.
Aniridia Treatment Market Share
The Aniridia Treatment industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Siemens Healthineers (Germany)
- Abbott (U.S.)
- General Electric Company (U.S.)
- BD (U.S.)
- Stryker (U.S.)
- Boston Scientific Corporation (U.S.)
- Cardinal Health (U.S.)
- Intuitive Surgical (U.S.)
- Cook (U.S.)
- Olympus Corporation (Japan)
- Johnson & Johnson Services Inc. (U.S.)
- Fresenius Medical Care AG & Co. KGaA (Germany)
- Baxter (U.S.)
- Richard Wolf GmbH (Germany)
- Dornier MedTech (Germany)
- KARL STORZ SE & Co. KG (Germany)
- Endo Pharmaceuticals Inc. (U.S.)
- HealthTronics Inc. (U.S.)
- MEDI TECH DEVICES PVT LTD (India)
- Coloplast Corp (U.S.)
Latest Developments in Global Aniridia Treatment Market
- In July 2021, Boston Scientific announced the launch of its LithoVue Empower Retrieval Deployment Device, a minimally invasive device designed to simplify the retrieval of kidney stones during ureteroscopic lithotripsy procedures
- In June 2021, Cook Medical received FDA clearance for its Zenith Alpha thoracic endovascular grafting system. This device is used for the minimally invasive repair of thoracic aortic aneurysms and dissections
- In May 2021, Olympus Corporation launched its Soltive Super Pulsed Laser System for urology procedures. This laser system offers enhanced precision and control for the treatment of kidney stones and benign prostatic hyperplasia
- In May 2021, Teleflex announced the FDA clearance of its UroLift Advanced Tissue Control (ATC) System for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. The UroLift ATC system provides improved tissue manipulation and control during the procedure
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.