Global Anti Infective Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
135.00 Billion
USD
184.70 Billion
2022
2030
USD
135.00 Billion
USD
184.70 Billion
2022
2030
| 2023 –2030 | |
| USD 135.00 Billion | |
| USD 184.70 Billion | |
|
|
|
|
Anti-Infective Drugs Market Analysis and Size
Anti-infective drugs have been a primary segment for several pharmaceutical companies for decades. Since the last two decades, the infection rate has increased considerably, mainly across the low and middle-income countries. Several advances in the formulation such as fixed-dose combination and increasing demand of diseases specific treatment are some of the major factors for the demand of antiviral drugs. The anti-infective drugs market is anticipated to grow hugely due to the increasing incidence of targeted diseases and awareness initiatives on anti-infective drugs.
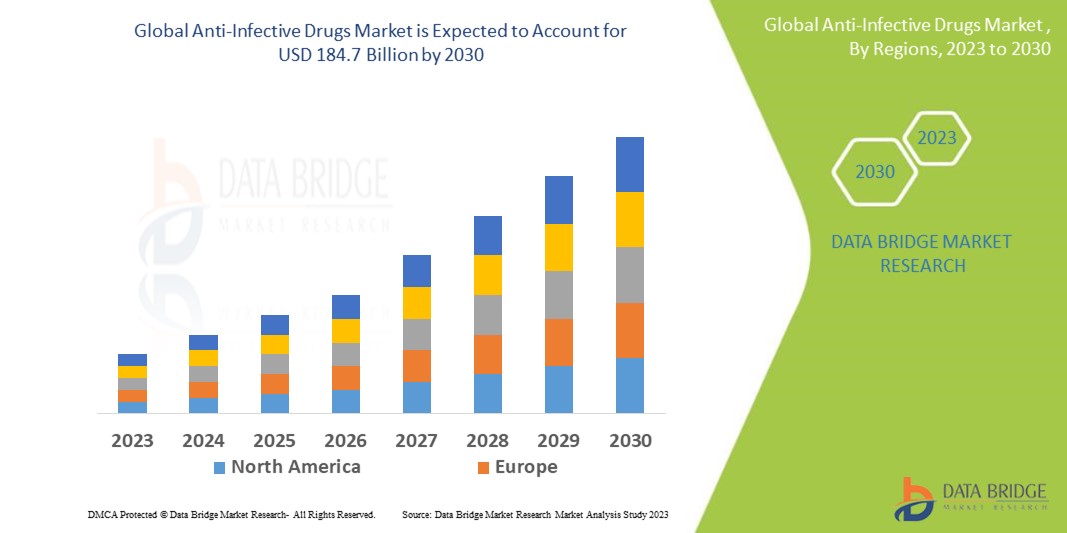
Data Bridge Market Research analyses a growth rate in the anti-infective drugs market in the forecast period 2023-2030. The expected CAGR of anti-infective drugs market is tend to be around 4% in the mentioned forecast period. The market was valued at USD 135 billion in 2022, and it would grow upto USD 184.7 billion by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Anti-Infective Drugs Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Antibiotics, Antivirals, Antifungals, Others), Indication (HIV Infections, Pneumonia, Hepatitis, Herpes Simplex Virus, Influenza, Others), Route of Administration (Oral, Topical, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Takeda Pharmaceutical Company Limited (Japan), Johnsons & Johnsons Services Inc (U.S.), Boehringer Ingelheim International GmbH (Germany), Abbvie, Inc (U.S.), Sun Pharmaceutical Industries Ltd. (India), Sanofi (France), GSK Plc. (U.K.), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Amneal Pharmaceuticals LLC. (U.S.), Alvogen (U.S), and Hikma Pharmaceuticals PLC (U.K.), Amneal Pharmaceuticals LLC. (U.S.), Alembic Pharmaceuticals Limited (India), Zydus Group (India) |
|
Market Opportunities |
|
Market Definition
Anti-infective drugs belongs to a class of therapeutics which causes suppression of muscle spasms. These type of drugs help in the stimulation of smooth muscle relaxation especially in the gastrointestinal tract. Antispasmodic medications generally treat symptoms such as stomach pain and cramping. They are most commonly used for the treatment of irritable bowel syndrome symptoms.
Global Anti-Infective Drugs Market Dynamics
Drivers
- Increasing Awareness About Infectious Diseases
There has been major growth in the number of multidrug-resistant organisms across numerous parts of the world, that further surges the importance of innovation in the anti-infective agents. Numerous institutes have established specific initiatives or campaigns to increase the awareness about infectious diseases among the public. For instance, the World Health Organization performed the World Antimicrobial Awareness Week (WAAW) with the theme, ‘Spread Awareness, Stop Resistance’ in November 2021 to raise awareness about antimicrobial resistance. Thus, this awareness initiatives boost the market growth.
- Growing Demand for Oral Drugs
Oral drugs is estimated to increase the market growth. The segment is expected to rise the global market as many of the products are available in capsule form and tablet form and it is a very common and convenient route of administration.
Opportunities
- Launch of Anti-infective Drugs
Major market players are releasing many products that are helping in boosting the growth of the market. For instance, in February 2021, Pfizer announced the launch of the C. difficile Awareness Initiative. C. difficile is an infectious bacterium that can cause a serious and potentially life-threatening infection related with symptoms from diarrhea to severe intestinal inflammation. The article specified that an estimate of about 462,000 C. difficile infection cases were being registered in the U.S. each year. The growing number of initiatives by several organizations and major market players on spreading awareness regarding infectious diseases are projected to boost the market growth. Furthermore, Xellia Pharmaceuticals, which is a pharmaceutical manufacturer specializing in anti-infective treatments, in August 2021, opened its manufacturing site in Cleveland, Ohio in U.S. The company released the first anti-infectives which is manufactured at the site for distribution to U.S. hospitals. Such activities are projected to increase the market growth during the forecast period.
Restraints/Challenges
- Lack of skilled professionals
The huge lack of qualified personnel who are not aware of the drugs options to give patients to could hamper the growth of the global anti-infective drugs market during the forecast period 2023-2030.
- Side-effects of anti-infective drugs
Several side effects such as weakness, dizziness, blurred vision, rashes, nausea, dry mouth, and abdominal bloating hamper the market growth. Any of the drug use is restricted because of the government developed guidelines to monitor how and where the medications are utilized.
This anti-infective drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the anti-infective drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Anti-Infective Drugs Market Scope
The anti-infective drugs market is segmented on the basis of product type, indication, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Antibiotics
- Antivirals
- Antifungals
- Others
Indication
- HIV Infections
- Pneumonia
- Hepatitis
- Herpes Simplex Virus
- Influenza
- Others
Route of Administration
- Oral
- Topical
- Parenteral
- Others
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Anti-Infective Drugs Market Regional Analysis/Insights
The anti-infective drugs market is analyzed and market size insights and trends are provided by product type, indication, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the anti-infective drugs market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America has been witnessing a positive growth for anti-infective drugs market throughout the forecasted period because of the sophisticated healthcare infrastructure, increasing awareness in society, well-established distribution channels, and a huge presence of e-commerce across the U.S. and Canada.
Asia-Pacific dominates the market because of the increasing attention of major market players to establish numerous emerging economics with significant growth. With the opening of several new hospitals and pharmacies in this area, the market is expanding.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Anti-Infective Drugs Market Share Analysis
The anti-infective drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to anti-infective drugs market
Key players operating in the anti-infective drugs market include:
- Takeda Pharmaceutical Company Limited (Japan)
- Johnsons & Johnsons Services Inc (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Abbvie, Inc (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Sanofi (France)
- GSK Plc. (U.K.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Amneal Pharmaceuticals LLC. (U.S.)
- Alvogen (U.S)
- Hikma Pharmaceuticals PLC (U.K.)
- Alembic Pharmaceuticals Limited (India)
- Zydus Group (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ANTI-INFECTIVE DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ANTI-INFECTIVE DRUGS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ANTI-INFECTIVE DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES MODEL
6 INDUSTRY INSIGHTS
7 EPIDEMIOLOGY
7.1 DISEASE PREVALENCE BY COUNTRY
7.2 DISEASE INCIDENCE BY COUNTRY
7.3 RISK FACTORS
(FACTORS THAT INCREASE THE LIKELIHOOD OF DEVELOPING A DISEASE, SUCH AS AGE, GENDER, LIFESTYLE FACTORS, ENVIRONMENTAL EXPOSURES, AND GENETIC PREDISPOSITION)
7.4 HEALTHCARE UTILIZATION
(HOW THE DISEASE AFFECTS HEALTHCARE UTILIZATION, SUCH AS HOSPITALIZATIONS, EMERGENCY ROOM VISITS, AND OUTPATIENT VISITS)
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIAL STATUS
9.1.1 CANDIDATE/AGENT
9.1.2 PROPERTIES
9.1.3 COMPANY NAME
9.1.4 THERAPEUTIC AREA
9.1.5 PRODUCT NAME
9.1.6 GENERIC NAME
9.1.7 TYPE
9.1.8 RESEARCH CODE
9.1.9 INDICATION
9.1.10 ORIGINATOR
9.1.11 SPONSOR
9.1.12 COLLABORATOR
9.1.13 LICENSOR
9.1.14 LICENSEE
9.2 DISTRIBUTION OF PRODUCTS BY PHASE
9.2.1 PRECLINICAL/RESEARCH PROJECT
9.2.2 PHASE I
9.2.3 PHASE II
9.2.4 PHASE III
9.2.5 PHSE IV
9.3 NUMBER OF SUBJECTS IN CLINICAL TRIALS
9.3.1 PHASE I
9.3.2 PHASE II
9.3.3 PHASE III
9.4 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
9.4.1 PRECLINICAL/RESEARCH PROJECT
9.4.2 PHASE I
9.4.3 PHASE II
9.4.4 PHASE III
9.4.5 PHASE IV
9.5 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
9.5.1 PRECLINICAL/RESEARCH PROJECT
9.5.2 PHASE I
9.5.3 PHASE II
9.5.4 PHASE III
9.5.5 PHASE IV
9.6 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
9.6.1 PRECLINICAL/RESEARCH PROJECT
9.6.2 PHASE I
9.6.3 PHASE II
9.6.4 PHASE III
9.6.5 PHASE IV
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.12.1 FORECAST MARKET OUTLOOK
10.12.2 CROSS COMPETITION
10.12.3 THERAPEUTIC PORTFOLIO
10.12.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 PATENT ANALYSIS
13.1 PATENT LANDSCAPE
13.2 USPTO NUMBER
13.3 PATENT EXPIRY
13.3.1 US PATENT EXPIRY
13.3.2 EUROPE PATENT EXPIRY
13.4 EPIO NUMBER
13.5 PATENT STRENGTH AND QUALITY
13.6 PATENT CLAIMS
13.7 PATENT CITATIONS
13.8 PATENT LITIGATION AND LICENSING
13.9 FILE OF PATENT
13.1 PATENT RECEIVED CONTRIES
13.11 TECHNOLOGY BACKGROUND
14 COMPETITIVE INTELLIGENCE TRACKING DATA
14.1 CLINICAL TRIAL MONITORING ANALYSIS
14.2 REGULATORY AND COMMERCIAL MONITORING ANALYSIS
15 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY PRODUCT TYPE
(NOTE: MARKET VALUE, MARKET VOLUME AND ASP WILL BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS )
15.1 OVERVIEW
15.2 ANTIBIOTICS DRUGS
15.2.1 CEPHALOSPORINS
15.2.1.1. CEPHALEXIN
15.2.1.1.1. MARKET VALUE (USD MILLION)
15.2.1.1.2. MARKET VOLUME (UNITS)
15.2.1.1.3. AVERAGE SELLING PRICE (USD)
15.2.1.2. CEFTRIAXONE
15.2.1.3. CEFUROXIME
15.2.1.4. CEFTAZIDIME
15.2.1.5. CEFIXIME
15.2.1.6. OTHER
15.2.2 PENICILLIN
15.2.2.1. AMOXICILLIN
15.2.2.2. AMPICILLIN
15.2.2.3. PENICILLIN G
15.2.2.4. METHICILLIN
15.2.2.5. AUGMENTIN
15.2.2.6. OTHER
15.2.3 FLUOROQUINOLONES
15.2.3.1. CIPROFLOXACIN
15.2.3.2. LEVOFLOXACIN
15.2.3.3. MOXIFLOXACIN
15.2.3.4. NORFLOXACIN
15.2.3.5. OTHER
15.2.4 MACROLIDES
15.2.4.1. AZITHROMYCIN
15.2.4.2. CLARITHROMYCIN
15.2.4.3. ERYTHROMYCIN
15.2.4.4. OTHER
15.2.5 CARBAPENEM
15.2.5.1. IMIPENEM
15.2.5.2. MEROPENEM
15.2.5.3. DORIPENEM
15.2.5.4. OTHER
15.2.6 TETRACYCLINES
15.2.6.1. DOXYCYCLINE
15.2.6.2. TETRACYCLINE
15.2.6.3. MINOCYCLINE
15.2.6.4. OTHER
15.2.7 AMINOGLYCOSIDES
15.2.7.1. GENTAMICIN
15.2.7.2. TOBRAMYCIN
15.2.7.3. AMIKACIN
15.2.7.4. STREPTOMYCIN
15.2.7.5. OTHER
15.2.8 GLYCOPEPTIDES
15.2.8.1. VANCOMYCIN
15.2.8.2. TEICOPLANIN
15.2.8.3. OTHER
15.2.9 OXAZOLIDINONES
15.2.9.1. LINEZOLID
15.2.9.2. OTHER
15.2.10 SULFONAMIDES
15.2.10.1. TRIMETHOPRIM-SULFAMETHOXAZOLE (TMP-SMX)
15.2.10.2. OTHER
15.2.11 OTHERS
15.2.11.1. CLINDAMYCIN
15.2.11.2. METRONIDAZOLE
15.2.11.3. NITROFURANTOIN
15.2.11.4. RIFAMPIN
15.2.11.5. OTHER
15.3 ANTIVIRALS DRUGS
15.3.1 NUCLEOSIDE/NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS (NRTIS)
15.3.1.1. LAMIVUDINE
15.3.1.2. ZIDOVUDINE
15.3.1.3. TENOFOVIR DISOPROXIL FUMARATE
15.3.1.4. EMTRICITABINE
15.3.1.5. ABACAVIR
15.3.1.6. OTHER
15.3.2 NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS (NNRTIS)
15.3.2.1. EFAVIRENZ
15.3.2.2. NEVIRAPINE
15.3.2.3. DELAVIRDINE
15.3.2.4. RILPIVIRINE
15.3.2.5. OTHER
15.3.3 PROTEASE INHIBITORS (PIS)
15.3.3.1. RITONAVIR
15.3.3.2. SAQUINAVIR
15.3.3.3. LOPINAVIR/RITONAVIR
15.3.3.4. ATAZANAVIR
15.3.3.5. DARUNAVIR
15.3.3.6. OTHER
15.3.4 INTEGRASE INHIBITORS
15.3.4.1. RALTEGRAVIR
15.3.4.2. DOLUTEGRAVIR
15.3.4.3. ELVITEGRAVIR
15.3.4.4. OTHER
15.3.5 FUSION INHIBITORS
15.3.5.1. ENFUVIRTIDE
15.3.5.2. IBALIZUMAB
15.3.5.3. OTHER
15.3.6 CCR5 ANTAGONISTS
15.3.6.1. MARAVIROC
15.3.6.2. OTHER
15.3.7 NEURAMINIDASE INHIBITORS
15.3.7.1. OSELTAMIVIR
15.3.7.2. ZANAMIVIR
15.3.7.3. PERAMIVIR
15.3.7.4. BALOXAVIR MARBOXIL
15.3.7.5. OTHER
15.3.8 POLYMERASE INHIBITORS (HCV)
15.3.8.1. SOFOSBUVIR
15.3.8.2. LEDIPASVIR-SOFOSBUVIR
15.3.8.3. GLECAPREVIR-PIBRENTASVIR
15.3.8.4. DACLATASVIR
15.3.8.5. OTHER
15.3.9 NEURAMINIDASE INHIBITORS (INFLUENZA)
15.3.9.1. OSELTAMIVIR
15.3.9.2. ZANAMIVIR
15.3.9.3. PERAMIVIR
15.3.9.4. BALOXAVIR MARBOXIL
15.3.9.5. OTHER
15.3.10 OTHER
15.4 ANTIFUNGAL
15.4.1 AZOLES
15.4.1.1. FLUCONAZOLE
15.4.1.2. ITRACONAZOLE
15.4.1.3. VORICONAZOLE
15.4.1.4. POSACONAZOLE
15.4.1.5. KETOCONAZOLE
15.4.1.6. OTHER
15.4.2 POLYENES
15.4.2.1. AMPHOTERICIN B
15.4.2.2. NYSTATIN
15.4.2.3. OTHER
15.4.3 ECHINOCANDINS
15.4.3.1. CASPOFUNGIN
15.4.3.2. MICAFUNGIN
15.4.3.3. ANIDULAFUNGIN
15.4.3.4. OTHER
15.4.4 ALLYLAMINES
15.4.4.1. TERBINAFINE
15.4.4.2. NAFTIFINE
15.4.4.3. OTHER
15.4.5 PYRIMIDINE ANALOGS
15.4.5.1. FLUCYTOSINE
15.4.5.2. GRISEOFULVIN
15.4.5.3. OTHER
15.4.6 TOPICAL ANTIFUNGALS
15.4.6.1. CLOTRIMAZOLE
15.4.6.2. MICONAZOLE
15.4.6.3. ECONAZOLE
15.4.6.4. KETOCONAZOLE
15.4.6.5. TERBINAFINE
15.4.6.6. OTHER
15.4.7 ANTIFUNGAL ANTIBIOTICS
15.4.7.1. NATAMYCIN
15.4.7.2. AMPHOTERICIN B
15.4.7.3. OTHER
15.4.8 TRIAZOLES
15.4.8.1. ISAVUCONAZOLE
15.4.8.2. RAVUCONAZOLE
15.4.8.3. ALBACONAZOLE
15.4.9 OTHER ANTIFUNGAL AGENTS:
15.4.9.1. CICLOPIROX
15.4.9.2. TAVABOROLE
15.4.9.3. EFINACONAZOLE
15.4.9.4. OTHER
15.5 ANTIPARASITIC DRUGS
15.5.1 ANTIMALARIAL DRUGS
15.5.1.1. CHLOROQUINE
15.5.1.2. ARTEMISININ AND ITS DERIVATIVES (ARTEMETHER, ARTESUNATE)
15.5.1.3. QUININE
15.5.1.4. MEFLOQUINE
15.5.1.5. ATOVAQUONE-PROGUANIL
15.5.1.6. PRIMAQUINE
15.5.1.7. DOXYCYCLINE
15.5.1.8. TAFENOQUINE
15.5.1.9. OTHER
15.5.2 ANTHELMINTICS
15.5.2.1. BENZIMIDAZOLES
15.5.2.1.1. ALBENDAZOLE
15.5.2.1.2. MEBENDAZOLE
15.5.2.1.3. OTHER
15.5.2.2. NICOTINIC AGONISTS
15.5.2.2.1. PYRANTEL PAMOATE
15.5.2.2.2. PYRVINIUM PAMOATE
15.5.2.2.3. OTHER
15.5.2.3. IVERMECTIN
15.5.2.4. PRAZIQUANTEL
15.5.2.5. DIETHYLCARBAMAZINE
15.5.2.6. OTHER
15.5.3 ANTIPROTOZOAL DRUGS
15.5.3.1. NITROIMIDAZOLES
15.5.3.1.1. METRONIDAZOLE
15.5.3.1.2. TINIDAZOLE
15.5.3.1.3. OTHER
15.5.3.2. QUINOLINES
15.5.3.2.1. CHLOROQUINE
15.5.3.2.2. HYDROXYCHLOROQUINE
15.5.3.2.3. OTHER
15.5.3.3. NITROFURANS
15.5.3.3.1. FURAZOLIDONE
15.5.4 PENTAMIDINE
15.5.5 ATOVAQUONE
15.5.6 OTHER
16 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY INDICATION
16.1 OVERVIEW
16.2 BACTERIAL INFECTIONS
16.2.1 PNEUMONIA
16.2.2 BRONCHITIS
16.2.3 SINUSITIS
16.2.4 CELLULITIS
16.2.5 WOUND INFECTIONS
16.2.6 GONORRHEA
16.2.7 SYPHILIS
16.2.8 CHLAMYDIA
16.2.9 PERITONITIS
16.2.10 DIVERTICULITIS
16.2.11 OSTEOMYELITIS
16.2.12 SEPTIC ARTHRITIS
16.2.13 BLOODSTREAM INFECTIONS (BACTEREMIA OR SEPSIS)
16.2.14 SURGICAL SITE INFECTIONS
16.2.15 MENINGITIS
16.2.16 DENTAL INFECTIONS
16.2.17 CONJUNCTIVITIS
16.2.18 KERATITIS
16.2.19 OTHER
16.3 VIRAL INFECTIONS
16.3.1 HUMAN IMMUNODEFICIENCY VIRUS (HIV) INFECTION
16.3.2 ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS)
16.3.3 INFLUENZA (FLU)
16.3.4 HERPES SIMPLEX VIRUS (HSV) INFECTIONS
16.3.5 VARICELLA-ZOSTER VIRUS (VZV) INFECTIONS
16.3.6 HEPATITIS B
16.3.7 HEPATITIS C
16.3.8 RESPIRATORY SYNCYTIAL VIRUS (RSV) INFECTION
16.3.9 HUMAN PAPILLOMAVIRUS (HPV) INFECTIONS
16.3.10 CYTOMEGALOVIRUS (CMV) INFECTIONS
16.3.11 OTHER
16.4 FUNGAL INFECTIONS
16.4.1 RAL THRUSH
16.4.2 VAGINAL YEAST INFECTION
16.4.3 RINGWORM
16.4.4 ATHLETE'S FOOT
16.4.5 CANDIDIASIS
16.4.6 ASPERGILLOSIS
16.4.7 CRYPTOCOCCAL MENINGITIS
16.4.8 OTHER
16.5 PARASITIC INFECTIONS
16.5.1 MALARIA
16.5.2 INTESTINAL WORM INFECTIONS
16.5.3 SCABIES
16.5.4 LICE INFESTATIONS
16.5.5 TOXOPLASMOSIS
16.5.6 TRYPANOSOMIASIS
16.5.7 LEISHMANIASIS
16.5.8 FILARIASIS (ELEPHANTIASIS)
16.5.9 OTHERS
17 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 ANTIBIOTICS
17.2.1.1. AMOXIL
17.2.1.2. ZITHROMAX
17.2.1.3. CIPRO
17.2.1.4. BIAXIN
17.2.1.5. CLEOCIN
17.2.1.6. OTHER
17.2.2 ANTIVIRAL
17.2.2.1. TAMIFLU
17.2.2.2. RELENZA
17.2.2.3. ZOVIRAX
17.2.2.4. VALTREX
17.2.2.5. FAMVIR
17.2.2.6. CYTOVENE
17.2.2.7. COPEGUS
17.2.2.8. SOVALDI
17.2.2.9. HARVONI
17.2.2.10. VEKLURY
17.2.2.11. OTHER
17.2.3 ANTIFUNGAL
17.2.3.1. SPORANOX
17.2.3.2. DIFLUCAN
17.2.3.3. VFEND
17.2.3.4. NOXAFIL
17.2.3.5. NIZORAL
17.2.3.6. FUNGIZONE
17.2.3.7. MYCOSTATIN
17.2.3.8. OTHER
17.2.4 ANTIPARASITIC DRUGS
17.2.4.1. ARALEN
17.2.4.2. LARIAM
17.2.4.3. STROMECTOL
17.2.4.4. ALBENZA
17.2.4.5. BILTRICIDE
17.2.4.6. MALARONE
17.2.4.7. FLAGYL
17.2.4.8. OTHER
17.3 GENERICS
18 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY MODE OF PURCHASE
18.1 OVERVIEW
18.2 PRESCRIPTION
18.3 OVER THE COUNTER
19 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY ROUTE OF ADMINISTRATION
19.1 OVERVIEW
19.2 ORAL
19.2.1 TABLET
19.2.2 CAPSULE
19.2.3 LIQUID
19.2.4 OTHER
19.3 PARENTERAL
19.3.1 INTRAMUSCULARLY
19.3.2 SUBCUTANEOUSLY
19.3.3 INTRAVENOUS
19.4 TOPICAL
19.4.1 CREAMS
19.4.2 OINTMENTS
19.4.3 POWDERS
19.4.4 SPRAYS
19.5 INTRAVAGINAL
19.6 RECTAL
19.7 OTHER
20 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY PATIENT TYPE
20.1 OVERVIEW
20.2 PEDIATRIC
20.3 ADULT
20.3.1 MALE
20.3.2 FEMALE
20.4 GERIATRIC
20.4.1 MALE
20.4.2 FEMALE
21 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITAL & CLINICS
21.2.1 PUBLIC
21.2.2 PRIVATE
21.3 SPECIALTY CENTRES
21.4 AMBULATORYSURGICAL CENTER
21.5 TRAUMA CENTERS
21.6 HOME HEALTHCARE
21.7 OTHERS
22 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAIL PAHRMACIES
22.3.1 HOSPITAL ASSOCIATED PHARMACY
22.3.2 RETAIL PHARMACY
22.3.3 ONLINE PHARMACY
22.3.4 OTHER
22.4 OTHERS
23 GLOBAL ANTI-INFECTIVE DRUGS MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL ANTI-INFECTIVE DRUGS MARKET, BY GEOGRAPHY
GLOBAL ANTI-INFECTIVE DRUGS MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 NORTH AMERICA
24.1.1 U.S.
24.1.2 CANADA
24.1.3 MEXICO
24.2 EUROPE
24.2.1 GERMANY
24.2.2 U.K.
24.2.3 ITALY
24.2.4 FRANCE
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 SWITZERLAND
24.2.8 TURKEY
24.2.9 BELGIUM
24.2.10 NETHERLANDS
24.2.11 DENMARK
24.2.12 SWEDEN
24.2.13 POLAND
24.2.14 NORWAY
24.2.15 FINLAND
24.2.16 REST OF EUROPE
24.3 ASIA-PACIFIC
24.3.1 JAPAN
24.3.2 CHINA
24.3.3 SOUTH KOREA
24.3.4 INDIA
24.3.5 SINGAPORE
24.3.6 THAILAND
24.3.7 INDONESIA
24.3.8 MALAYSIA
24.3.9 PHILIPPINES
24.3.10 AUSTRALIA
24.3.11 NEW ZEALAND
24.3.12 VIETNAM
24.3.13 TAIWAN
24.3.14 REST OF ASIA-PACIFIC
24.4 SOUTH AMERICA
24.4.1 BRAZIL
24.4.2 ARGENTINA
24.4.3 PERU
24.4.4 REST OF SOUTH AMERICA
24.5 MIDDLE EAST AND AFRICA
24.5.1 SOUTH AFRICA
24.5.2 EGYPT
24.5.3 BAHRAIN
24.5.4 UNITED ARAB EMIRATES
24.5.5 KUWAIT
24.5.6 OMAN
24.5.7 QATAR
24.5.8 SAUDI ARABIA
24.5.9 REST OF MEA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL ANTI-INFECTIVE DRUGS MARKET, SWOT AND DBMR ANALYSIS
26 GLOBAL ANTI-INFECTIVE DRUGS MARKET, COMPANY PROFILE
26.1 JOHNSONS & JOHNSONS SERVICES INC
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPEMNTS
26.2 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPEMNTS
26.3 ABBVIE, INC
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPEMNTS
26.4 SUN PHARMACEUTICAL INDUSTRIES LTD.
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPEMNTS
26.5 SANOFI
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPEMNTS
26.6 GSK PLC.
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPEMNTS
26.7 NOVARTIS AG
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPEMNTS
26.8 PFIZER INC.
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPEMNTS
26.9 HIKMA PHARMACEUTICALS PLC
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPEMNTS
26.1 AMNEAL PHARMACEUTICALS LLC.
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPEMNTS
26.11 ALEMBIC PHARMACEUTICALS LIMITED
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPEMNTS
26.12 ZYDUS GROUP
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPEMNTS
26.13 FRESENIUS KABI
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPEMNTS
26.14 MERCK & CO., INC.
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPEMNTS
26.15 ROCHE HOLDING AG
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPEMNTS
26.16 ASTRAZENECA PLC
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPEMNTS
26.17 BAYER AG
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPEMNTS
26.18 ELI LILLY AND COMPANY
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPEMNTS
26.19 GILEAD SCIENCES, INC.
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPEMNTS
26.2 BRISTOL MYERS SQUIBB COMPANY
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPEMNTS
26.21 ASTELLAS PHARMA INC.
26.21.1 COMPANY OVERVIEW
26.21.2 REVENUE ANALYSIS
26.21.3 GEOGRAPHIC PRESENCE
26.21.4 PRODUCT PORTFOLIO
26.21.5 RECENT DEVELOPEMNTS
26.22 TAKEDA PHARMACEUTICAL COMPANY LIMITED
26.22.1 COMPANY OVERVIEW
26.22.2 REVENUE ANALYSIS
26.22.3 GEOGRAPHIC PRESENCE
26.22.4 PRODUCT PORTFOLIO
26.22.5 RECENT DEVELOPEMNTS
26.23 CIPLA LTD
26.23.1 COMPANY OVERVIEW
26.23.2 REVENUE ANALYSIS
26.23.3 GEOGRAPHIC PRESENCE
26.23.4 PRODUCT PORTFOLIO
26.23.5 RECENT DEVELOPEMNTS
26.24 LUPIN
26.24.1 COMPANY OVERVIEW
26.24.2 REVENUE ANALYSIS
26.24.3 GEOGRAPHIC PRESENCE
26.24.4 PRODUCT PORTFOLIO
26.24.5 RECENT DEVELOPEMNTS
26.25 GLENMARK PHARMACEUTICALS LTD.
26.25.1 COMPANY OVERVIEW
26.25.2 REVENUE ANALYSIS
26.25.3 GEOGRAPHIC PRESENCE
26.25.4 PRODUCT PORTFOLIO
26.25.5 RECENT DEVELOPEMNTS
26.26 DR. REDDY'S LABORATORIES LTD.
26.26.1 COMPANY OVERVIEW
26.26.2 REVENUE ANALYSIS
26.26.3 GEOGRAPHIC PRESENCE
26.26.4 PRODUCT PORTFOLIO
26.26.5 RECENT DEVELOPEMNTS
26.27 REGENERON PHARMACEUTICALS, INC.
26.27.1 COMPANY OVERVIEW
26.27.2 REVENUE ANALYSIS
26.27.3 GEOGRAPHIC PRESENCE
26.27.4 PRODUCT PORTFOLIO
26.27.5 RECENT DEVELOPEMNTS
26.28 JIANGSU HENGRUI MEDICINE CO., LTD.
26.28.1 COMPANY OVERVIEW
26.28.2 REVENUE ANALYSIS
26.28.3 GEOGRAPHIC PRESENCE
26.28.4 PRODUCT PORTFOLIO
26.28.5 RECENT DEVELOPEMNTS
26.29 GENETECH
26.29.1 COMPANY OVERVIEW
26.29.2 REVENUE ANALYSIS
26.29.3 GEOGRAPHIC PRESENCE
26.29.4 PRODUCT PORTFOLIO
26.29.5 RECENT DEVELOPEMNTS
26.3 HETERO
26.30.1 COMPANY OVERVIEW
26.30.2 REVENUE ANALYSIS
26.30.3 GEOGRAPHIC PRESENCE
26.30.4 PRODUCT PORTFOLIO
26.30.5 RECENT DEVELOPEMNTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












