Global Antinuclear Antibody Test Market
Market Size in USD Billion
CAGR :
% 
 USD
3.00 Billion
USD
8.14 Billion
2024
2032
USD
3.00 Billion
USD
8.14 Billion
2024
2032
| 2025 –2032 | |
| USD 3.00 Billion | |
| USD 8.14 Billion | |
|
|
|
|
Anti-Nuclear Antibody Test Market Size
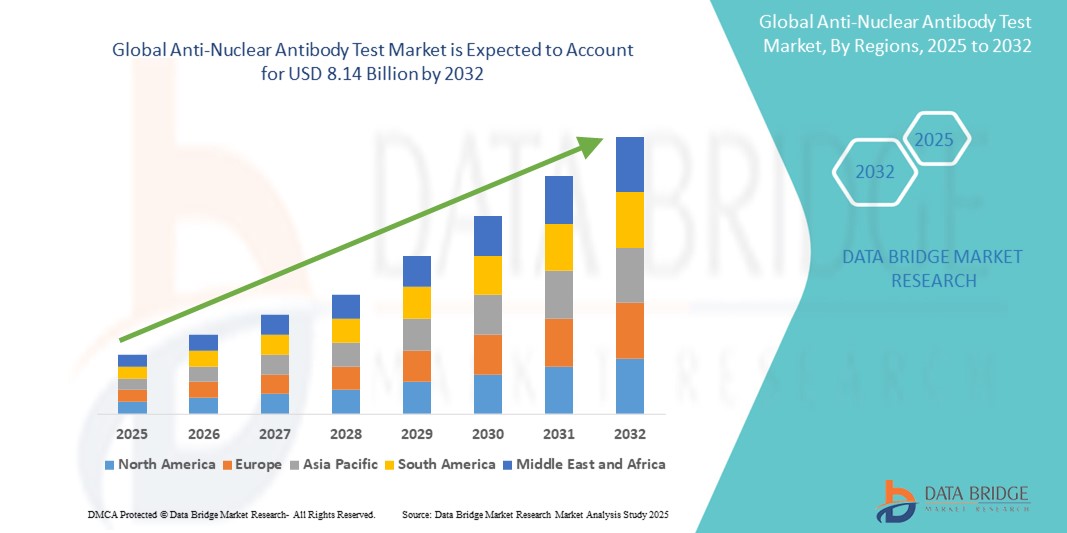
- The global anti-nuclear antibody test market size was valued at USD 3.00 billion in 2024 and is expected to reach USD 8.14 billion by 2032, at a CAGR of 13.30% during the forecast period
- This growth is driven by factors such as rising prevalence of autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, and Sjogren’s syndrome and increasing elderly population
Anti-Nuclear Antibody Test Market Analysis
- The anti-nuclear antibody test is a vital diagnostic method used to detect autoantibodies that attack components within the nucleus of cells, supporting the diagnosis of autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis. It is recognized for its high sensitivity, clinical utility, and ability to identify disease at an early stage, making it an essential tool in autoimmune disease diagnostics
- The anti-nuclear antibody test market is witnessing consistent growth, driven by the rising incidence of autoimmune diseases, increasing public awareness and routine screening initiatives, ongoing advancements in diagnostic technologies, and the expansion of healthcare infrastructure worldwide
- North America is expected to dominate the anti-nuclear antibody test market due to region’s advanced healthcare infrastructure and strong emphasis on early disease detection and personalized medicine
- Asia-Pacific is expected to be the fastest growing region in the anti-nuclear antibody test market during the forecast period due to increasing awareness of autoimmune diseases and growing demand for early diagnostic interventions
- Indirect Immunofluorescence (IIF) segment is expected to dominate the market due to its superior sensitivity and comprehensive diagnostic capabilities. IIF remains the gold standard method for ANA testing because it can detect a broad spectrum of autoantibodies associated with various autoimmune disorders, including systemic lupus erythematosus (SLE) and Sjögren's syndrome. Its ability to identify different staining patterns also helps clinicians better interpret results and differentiate between specific autoimmune conditions, which is crucial for accurate diagnosis and treatment planning
Report Scope and Anti-Nuclear Antibody Test Market Segmentation
|
Attributes |
Anti-Nuclear Antibody Test Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Anti-Nuclear Antibody Test Market Trends
“Growing Research Efforts Focused on Autoimmune Diseases”
- One prominent trend in the global anti-nuclear antibody test market is the growing research efforts focused on autoimmune diseases
- This trend is driven by the rising global burden of autoimmune disorders, heightened scientific interest in understanding disease mechanisms, and the push for more precise and early diagnostic tools
- For instance, organizations such as the National Institutes of Health (NIH) and pharmaceutical companies such as Roche and Eli Lilly are actively funding and conducting research aimed at identifying novel autoantibodies and improving diagnostic accuracy for conditions such as systemic lupus erythematosus and rheumatoid arthritis
- The emphasis on autoimmune disease research is growing across developed regions such as North America and Europe, as well as in emerging economies where healthcare investment and diagnostic capabilities are rapidly advancing
- As the scientific community continues to uncover deeper insights into autoimmunity, the demand for reliable and advanced anti-nuclear antibody testing is expected to grow, driving innovation and shaping the future landscape of autoimmune diagnostics
Anti-Nuclear Antibody Test Market Dynamics
Driver
“Expansion of Diagnostic Centers and Laboratories”
- The expansion of diagnostic centers and laboratories worldwide is significantly driving the growth of the global anti-nuclear antibody test market. As healthcare systems evolve and the demand for accurate and timely diagnostics increases, more facilities are being established to cater to this need
- These centers play a critical role in enhancing access to diagnostic testing, making it easier for patients to obtain necessary evaluations for autoimmune disorders
- With advancements in technology and improved laboratory capabilities, these facilities can now offer a broader range of tests, including anti-nuclear antibody tests, which are vital for the diagnosis of various autoimmune conditions
- In addition, the proliferation of specialized laboratories allows for faster turnaround times and more precise results, thereby boosting clinician confidence in diagnosis and treatment planning
- This shift is further supported by an increasing focus on early detection and preventive healthcare, which emphasizes the importance of comprehensive testing
For instance,
- In April 2024, according to the article published by Living Media India Limited., Apollo Health & Lifestyle, a subsidiary of Apollo Hospitals Enterprise Limited, aims to broaden its presence in India while maintaining its leadership in diagnostic and preventive care. This growth in diagnostic facilities will significantly enhance access to ANA testing, driving market expansion
- As healthcare systems advance, there is a greater demand for accurate and accessible diagnostic services, leading to the establishment of more facilities. This ongoing expansion of diagnostic centers and laboratories significantly supports the rise of the anti-nuclear antibody test market
Opportunity
“Integration of Digital Health Solutions”
- The integration of digital health solutions offers a number of opportunities for the global Anti-Nuclear Antibody (ANA) Test Market, in turn, transforming access and utilization of testing. One of the most significant benefits is the enhancement of patient access to diagnostic services.
- Telemedicine platforms have become increasingly popular, allowing patients to consult healthcare professionals remotely and receive recommendations for ANA testing without the barriers of geographical distance or the need for in-person visits
- This convenience can lead to higher testing rates, as patients are more likely to seek testing when it is easily accessible
For instance,
- Siemens Healthineers has introduced digital diagnostics platforms that integrate laboratory data, including autoantibody test results, into centralized digital health records to support faster clinical decision-making and improved patient outcome
- As healthcare systems continue to embrace digital transformation, the adoption of connected diagnostic technologies is expected to create new growth avenues for the anti-nuclear antibody test market
Restraint/Challenge
“Lack of Standardization for Testing Protocols”
- The lack of standardization for testing protocols in the Global Anti-Nuclear Antibody (ANA) Test Market acts as a significant restraint because it leads to inconsistencies in how the tests are conducted and interpreted across different laboratories and regions
- Varying methodologies, reagents, and result interpretation guidelines create discrepancies in the sensitivity and specificity of ANA tests, making it difficult for healthcare providers to rely on consistent outcomes
- This inconsistency expected to undermine the clinical utility of ANA tests, as physicians may struggle to make accurate diagnoses based on conflicting or unclear results. Moreover, without standardized testing protocols, it becomes challenging for manufacturers to develop universally accepted test kits, limiting global adoption
For instance,
- The use of indirect immunofluorescence (IIF) on HEp-2 cells may yield different results depending on the substrate quality, dilution cut-offs, and pattern interpretation criteria. While some laboratories follow guidelines from the International Consensus on ANA Patterns (ICAP), others do not, leading to discrepancies in test sensitivity and specificity
- The absence of standardized testing protocols in the Global Anti-Nuclear Antibody (ANA) Test Market hampers consistent test results, making it difficult for healthcare professionals to trust and utilize the test reliably. This inconsistency complicates diagnoses and slows down regulatory approvals and global adoption, limiting the test's overall effectiveness in clinical practice
Anti-Nuclear Antibody Test Market Scope
The market is segmented on the basis of antibody type, product, technique, application, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Antibody Type |
|
|
By Product |
|
|
By Technique |
|
|
By Application |
|
|
By End User |
|
|
By Distribution Channel |
|
In 2025, the Indirect Immunofluorescence (IIF) is projected to dominate the market with a largest share in technique segment
The Indirect Immunofluorescence (IIF) segment is expected to dominate the anti-nuclear antibody test market in 2025 due to its superior sensitivity and comprehensive diagnostic capabilities. IIF remains the gold standard method for ANA testing because it can detect a broad spectrum of autoantibodies associated with various autoimmune disorders, including systemic lupus erythematosus (SLE) and Sjögren's syndrome. Its ability to identify different staining patterns also helps clinicians better interpret results and differentiate between specific autoimmune conditions, which is crucial for accurate diagnosis and treatment planning.
The rheumatoid arthritis is expected to account for the largest share during the forecast period in application segment
In 2025, the rheumatoid arthritis segment is expected to dominate the market due to high prevalence of the disease globally and the strong clinical reliance on anti-nuclear antibody (ANA) testing for early and accurate diagnosis. Rheumatoid arthritis is a common autoimmune disorder that often requires routine monitoring and early detection to prevent joint damage and improve patient outcomes. The growing aging population, increased awareness, and advancements in diagnostic technologies further contribute to the segment's leading position in the ANA test market.
Anti-Nuclear Antibody Test Market Regional Analysis
“North America Holds the Largest Share in the Anti-nuclear antibody test Market”
- North America dominates the anti-nuclear antibody test market, driven by the region’s advanced healthcare infrastructure and strong emphasis on early disease detection and personalized medicine
- U.S. holds a significant share due to high prevalence of autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, along with robust healthcare spending and widespread awareness of diagnostic testing
- Regional leadership is further supported by the presence of leading diagnostic companies such as Thermo Fisher Scientific and Bio-Rad Laboratories, strong regulatory frameworks, and ongoing investment in research and development for autoimmune diagnostics
- With a growing focus on early diagnosis, adoption of advanced diagnostic platforms, and integration of digital health technologies, North America is expected to maintain its dominant position in the global anti-nuclear antibody test market through 2032
“Asia-Pacific is Projected to Register the Highest CAGR in the Anti-nuclear antibody test Market”
- Asia-Pacific is expected to witness the highest growth rate in the anti-nuclear antibody test market, driven by increasing awareness of autoimmune diseases and growing demand for early diagnostic interventions
- China holds a significant share due to a rapidly aging population, increasing prevalence of autoimmune disorders, and growing investment in diagnostic infrastructure and healthcare access
- The region’s market expansion is also supported by rising healthcare expenditures, government initiatives aimed at improving disease screening, and growing private sector involvement in diagnostic services
- With improved access to healthcare services, expanding insurance coverage, and rising demand for advanced diagnostic solutions, Asia-Pacific is poised to lead global market growth for anti-nuclear antibody testing through 2032
Anti-Nuclear Antibody Test Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Abcam Limited (U.K.)
- Antibodies Incorporated (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- BioVision Inc. (U.S.)
- Grifols, S.A. (Spain)
- Immuno Concepts Ltd. (U.S.)
- Merck KGaA (Germany)
- Orgentec Diagnostika GmbH (Germany)
- Revvity, Inc. (U.S.)
- Quidel Corporation (U.S.)
- Seramun Diagnostica GmbH (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Transasia Bio-Medicals Ltd. (India)
- Trinity Biotech plc (Ireland)
- Werfen S.A. (Spain)
- ZEUS Scientific, Inc. (U.S.)
Latest Developments in Global Anti-Nuclear Antibody Test Market
- In September 2024, A. Menarini Diagnostics and Nucleix have formed a strategic partnership to launch a non-invasive bladder cancer test in Europe. This collaboration aims to enhance early detection and diagnosis of bladder cancer, providing significant benefits to patients and healthcare providers. For Menarini, this partnership strengthens its portfolio and positions the company as a leader in innovative diagnostic solutions
- In May 2023, Thermo Fisher and BRIN have partnered to enhance research capabilities in Indonesia, focusing on advancing scientific innovation and collaboration in life sciences, biotechnology, and environmental studies for local researchers
- In January 2023, Revvity’s EUROIMMUN has launched an automated indirect immunofluorescence test (IIFT) system to enhance diagnostic accuracy and efficiency in detecting autoantibodies. This innovation streamlines laboratory workflows, reduces manual errors, and positions EUROIMMUN to meet growing demand, ultimately driving revenue growth and reinforcing Revvity’s commitment to transformative healthcare solutions
- In January 2023, Quantum-Si announced a collaboration with Aviva Systems Biology to develop protein enrichment kits for enhanced protein sequencing. The kits will include immunoprecipitation tools to streamline workflows and enable in-depth analysis of protein variants, facilitating research into biological processes and diseases
- In November 2022, Bio-Rad has expanded its range of quality controls specifically for Abbott's clinical diagnostics platforms, enhancing laboratory performance and reliability. This initiative improves diagnostic accuracy and patient care and also strengthens Bio-Rad's competitive position in the healthcare market. By providing innovative quality control solutions, Bio-Rad aims to meet the growing demands of laboratories, ultimately driving revenue growth and reinforcing its commitment to advancing diagnostic excellence
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.