Global Antisense Oligonucleotide For Neuromuscular Disorders Market
Market Size in USD Billion
CAGR :
% 
 USD
2.03 Billion
USD
4.87 Billion
2024
2032
USD
2.03 Billion
USD
4.87 Billion
2024
2032
| 2025 –2032 | |
| USD 2.03 Billion | |
| USD 4.87 Billion | |
|
|
|
|
Antisense Oligonucleotide for Neuromuscular Disorders Market Size
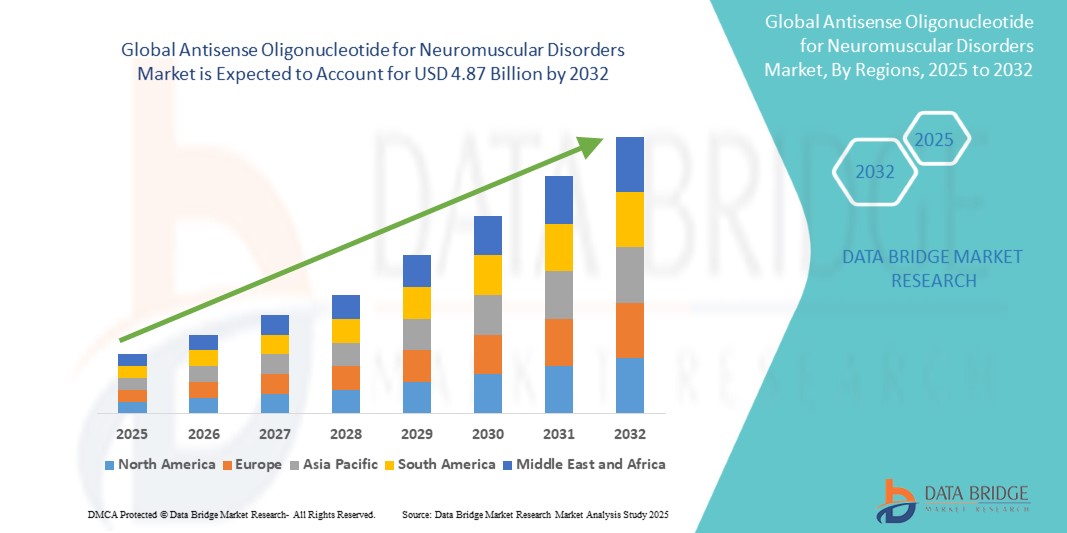
- The global antisense oligonucleotide for neuromuscular disorders market size was valued at USD 2.03 billion in 2024 and is expected to reach USD 4.87 billion by 2032, at a CAGR of 11.50% during the forecast period
- The market growth is primarily driven by the increasing prevalence of neuromuscular disorders globally, such as Duchenne muscular dystrophy (DMD), spinal muscular atrophy (SMA), and amyotrophic lateral sclerosis (ALS), which has created a critical demand for advanced and targeted therapeutic approaches such as antisense oligonucleotides (ASOs)
- Growing investments in genetic and RNA-based research, coupled with accelerated regulatory approvals for novel ASO-based drugs, are further propelling market expansion. The success of ASO therapies such as Spinraza and Exondys 51 has not only validated the efficacy of this treatment modality but also spurred pharmaceutical and biotech firms to increase their R&D activities in this area
Antisense Oligonucleotide for Neuromuscular Disorders Market Analysis
- Antisense oligonucleotides (ASOs) are emerging as a revolutionary therapeutic approach in the treatment of neuromuscular disorders, targeting the root cause of genetic abnormalities by altering RNA expression. These molecules are designed to modulate splicing or suppress mutant proteins, offering targeted precision therapy with fewer off-target effects
- The escalating demand for ASO-based therapies is primarily driven by the growing prevalence of rare neuromuscular diseases such as Duchenne Muscular Dystrophy (DMD), Amyotrophic Lateral Sclerosis (ALS), and Spinal Muscular Atrophy (SMA), coupled with increasing regulatory support for orphan drug development
- North America dominated the antisense oligonucleotide for neuromuscular disorders market with the largest revenue share of 45% in 2024, due to the presence of key pharmaceutical innovators, early FDA approvals (such as Spinraza, Exondys 51), and strong R&D funding. The U.S. in particular has witnessed accelerated clinical trial pipelines and market availability of approved ASO therapies
- Asia-Pacific is projected to be the fastest growing region in the antisense oligonucleotide for neuromuscular disorders market, with an expected CAGR of 20.4% from 2025 to 2032, attributed to increased investment in genomics, growing awareness of rare diseases, and improving healthcare infrastructure in countries such as China, India, and Japan
- The Duchenne Muscular Dystrophy (DMD) segment dominated the antisense oligonucleotide for neuromuscular disorders market with the largest revenue share of 43.6% in 2024, backed by regulatory approvals, commercialized treatments, and a strong clinical pipeline
Report Scope and Antisense Oligonucleotide for Neuromuscular Disorders Market Segmentation
|
Attributes |
Antisense Oligonucleotide for Neuromuscular Disorders Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Antisense Oligonucleotide for Neuromuscular Disorders Market Trends
Rising Demand Driven by Advances in Precision Medicine and Genetic Therapies
- A significant and accelerating trend in the global Antisense Oligonucleotide for Neuromuscular Disorders market is the growing focus on precision medicine, which emphasizes the development of targeted therapies based on genetic profiling and specific molecular pathways involved in neuromuscular diseases
- For instance, in May 2024, Sarepta Therapeutics expanded its pipeline by initiating clinical trials of a next-generation antisense oligonucleotide therapy aimed at enhancing exon skipping efficiency for Duchenne Muscular Dystrophy (DMD), indicating the industry’s emphasis on therapeutic specificity
- The development of advanced antisense chemistries such as phosphorodiamidate morpholino oligomers (PMOs) and locked nucleic acid (LNA) oligonucleotides has significantly improved the stability, specificity, and efficacy of treatments for genetic neuromuscular conditions. These innovations are enabling longer-lasting effects and reducing dosing frequency
- In addition, the increasing availability of genetic testing and improved diagnostic tools is facilitating earlier detection and patient stratification, which is essential for maximizing the efficacy of antisense therapies. This trend is particularly evident in regions such as North America and Europe where precision diagnostics are rapidly advancing
- Regulatory incentives, including fast-track approvals, orphan drug designations, and grants for rare disease research, are further accelerating the development and commercialization of antisense oligonucleotide therapies. These supportive frameworks are encouraging biopharmaceutical companies to invest in novel treatment modalities for unmet clinical needs
- As a result, the antisense oligonucleotide for neuromuscular disorders market is experiencing significant growth, supported by both technological innovation and a robust clinical pipeline addressing conditions such as spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), and myotonic dystrophy
Antisense Oligonucleotide for Neuromuscular Disorders Market Dynamics
Driver
Growing Demand for Precision Therapies in Neuromuscular Disorders
- The rising global burden of neuromuscular disorders (NMDs), including Duchenne Muscular Dystrophy (DMD), Spinal Muscular Atrophy (SMA), Amyotrophic Lateral Sclerosis (ALS), and other rare genetic conditions, is driving the need for targeted therapies. Antisense oligonucleotides (ASOs) are emerging as highly promising treatment options due to their ability to modulate gene expression with high precision
- For instance, in April 2024, Ionis Pharmaceuticals and Biogen jointly announced positive Phase III results for Tofersen, an ASO therapy targeting SOD1-related ALS, reinforcing the commercial and therapeutic viability of ASO-based interventions.
- Unlike conventional drugs, ASOs can be designed to silence, modify, or restore specific gene functions, making them exceptionally effective in addressing the root causes of genetic neuromuscular diseases. This precision medicine approach aligns with the ongoing trend in healthcare toward personalized and targeted therapies
- Furthermore, regulatory agencies such as the U.S. FDA and EMA are increasingly granting orphan drug status, fast track, and breakthrough designations to ASO-based products. These regulatory incentives are accelerating clinical development timelines and market access, encouraging biotech and pharmaceutical firms to increase R&D investments in the ASO space
- Increasing collaborations between academia, biotech startups, and large pharmaceutical companies are also fueling innovation. Investments in delivery technologies (such as lipid nanoparticles and conjugated ASOs) are helping improve tissue targeting, especially in hard-to-reach areas such as the central nervous system. This is broadening the scope of ASOs beyond SMA and DMD to other progressive NMDs
- Moreover, improved diagnostic tools and wider adoption of genetic testing and newborn screening programs are enabling earlier diagnosis and intervention—further boosting the demand for ASOs in treating neuromuscular disorders effectively and at earlier stages
Restraint/Challenge
High Development Costs, Complex Delivery Mechanisms, and Limited Accessibility
- Despite their therapeutic promise, antisense oligonucleotides face substantial challenges related to high development costs, complex delivery systems, and limited access in low- and middle-income countries. Developing ASO therapies involves extensive R&D investment, sophisticated synthesis processes, and specialized clinical trials that drive up the overall cost of drug development
- The average development cost for a single ASO drug can exceed hundreds of millions of dollars, and this financial burden is further amplified in rare diseases where patient populations are small and trials are more difficult to scale. This limits market participation mostly to large pharmaceutical companies or well-funded biotech firms
- In addition, the delivery of ASOs to specific tissues, especially to muscle or the central nervous system, poses a major technical barrier. Current intrathecal or intravenous delivery methods are invasive and may require repeated administration. Moreover, off-target effects, immune responses, and potential toxicity can complicate clinical outcomes and raise regulatory scrutiny
- There is also an unmet need for scalable, non-invasive, and cost-effective delivery platforms that can enhance bioavailability while minimizing side effects. Companies are exploring advanced approaches such as peptide conjugates, nanoparticles, and viral vectors, but these solutions are still under development and face their own safety and scalability challenges
- Furthermore, healthcare reimbursement and access issues hinder ASO adoption, especially in emerging markets. The high cost of therapy—often reaching several hundred thousand dollars per patient annually—limits patient access even in developed markets unless backed by insurance or government support
- Lastly, regulatory hurdles, particularly in jurisdictions with less familiarity with gene-targeted therapies, may delay product approvals and commercialization. Ensuring global harmonization of regulatory pathways and educating clinicians and patients about ASO benefits are necessary steps to broaden access and overcome skepticism
Antisense Oligonucleotide for Neuromuscular Disorders Market Scope
The market is segmented on the basis of type, delivery method, mechanism of action, and application.
• By Type
On the basis of type, the antisense oligonucleotide for neuromuscular disorders market is segmented into DNA-based Antisense Oligonucleotides, RNA-based Antisense Oligonucleotides, Locked Nucleic Acid (LNA) Oligonucleotides, Phosphorodiamidate Morpholino Oligomers (PMOs), and others. DNA-based antisense oligonucleotides dominated the market, holding the largest revenue share of 36.4% in 2024, thanks to their well-established efficacy demonstrated across both preclinical and early clinical studies. These molecules are known for their robust binding to target mRNA sequences, leading to reliable gene knockdown or modulation in diverse neuromuscular models.
Phosphorodiamidate Morpholino Oligomers (PMOs) are forecast to register the highest CAGR of 22.8% from 2025 to 2032, owing to their enhanced stability, minimal off-target effects, and proven success with FDA-approved therapies (e.g., for Duchenne Muscular Dystrophy). PMOs resist enzymatic degradation and maintain binding affinity even in harsh biological environments, making them suitable for in vivo therapeutic use. Their safety profile and emerging delivery technologies, including peptide conjugation and nanoparticle carriers, are further propelling their uptake. As clinical development shifts toward durable and safe ASO platforms, PMOs are set to gain greater traction across disease indications.
• By Delivery Method
On the basis of delivery method, the antisense oligonucleotide for neuromuscular disorders market is segmented into Intravenous, subcutaneous, intrathecal, inhalation-based, and others. Intrathecal delivery claimed the largest share at 41.7% in 2024, due to its direct administration into the cerebrospinal fluid, offering superior central nervous system (CNS) targeting—critical for treating disorders like Spinal Muscular Atrophy (SMA). This method bypasses the blood–brain barrier, achieving therapeutic ASO concentrations within spinal motor neurons. The growing clinical success of therapies delivered via intrathecal injection reinforces its clinical preference.
Inhalation-based delivery is projected to grow fastest with a CAGR of 21.3% from 2025 to 2032, driven by advances in aerosolized delivery systems and increasing interest in treating respiratory-associated neuromuscular dysfunction. This approach offers non-invasive administration, which may improve patient compliance—especially in pediatric or outpatient settings. Innovations in nebulizer technologies and formulation science are also enabling stable aerosolization of ASO molecules. Furthermore, exploration into targeting respiratory muscles and neural pathways via inhaled therapies opens new treatment avenues within neuromuscular medicine, marking inhalation as a rapidly emergent delivery strategy.
• By Mechanism of Action
On the basis of mechanism of action, the antisense oligonucleotide for neuromuscular disorders market is segmented into exon skipping, splice modulation, gene silencing, translation inhibition, and others. Exon Skipping led the market with a 38.9% share in 2024, led by successful therapeutic examples targeting DMD exon mutations. This mechanism allows precise exclusion of pathogenic exons, restoring functional protein reading frames and enabling partial restoration of dystrophin expression. The established regulatory approvals and clinical adoption of exon-skipping ASOs boost its prominence.
Splice Modulation is on track to grow at the fastest CAGR of 20.5% from 2025 to 2032, thanks to its ability to correct aberrant splicing—central to diverse neuromuscular diseases like ALS and myotonic dystrophy. This mechanism provides versatility in targeting a range of splicing mutations and enhancing therapeutic reach. Ongoing advances in splicing biology and improved ASO design tools are facilitating more effective and selective modulation. As more disorders are identified as splicing-related, and as regulatory bodies prioritize genetic precision therapies, splice modulation is gaining substantial interest from pharma and biotech players alike.
• By Application
On the basis of application, the antisense oligonucleotide for neuromuscular disorders market is segmented into Duchenne Muscular Dystrophy (DMD), Spinal Muscular Atrophy (SMA), Amyotrophic Lateral Sclerosis (ALS), Myotonic Dystrophy, and Others. Duchenne Muscular Dystrophy (DMD) dominated with the largest revenue share of 43.6% in 2024, sustained by multiple regulatory-approved ASO therapies and a strong clinical development pipeline. High unmet needs, well-characterized genetic targets, and favorable reimbursement pathways support continued investment. Public awareness and advocacy funding for DMD also accelerate market momentum.
Spinal Muscular Atrophy (SMA) is expected to register the fastest growth with a projected CAGR of 23.2% from 2025 to 2032, supported by increasing availability of intrathecal delivery infrastructure and supportive reimbursement frameworks for rare disease treatments. Early success in prolonging survival and motor function improvements for SMA patients drives continued pipeline investment. Additionally, newborn screening programs and therapeutic awareness campaigns are expanding early diagnosis, fueling demand for effective ASO interventions in this disease category.
Antisense Oligonucleotide for Neuromuscular Disorders Market Regional Analysis
- North America dominated the antisense oligonucleotide for neuromuscular disorders market with the largest revenue share of 45% in 2024, driven by robust R&D capabilities, strong presence of leading biotech companies, and favorable regulatory support for rare disease treatments. The increasing number of clinical trials, particularly for Duchenne Muscular Dystrophy (DMD) and Spinal Muscular Atrophy (SMA), along with fast-track approvals by the FDA, are accelerating product commercialization across the region
- The region’s growth is further bolstered by rising healthcare expenditure, high awareness of neuromuscular disorders, and active collaborations between academia, pharmaceutical firms, and government bodies
- The expanding investment in gene and RNA therapies, particularly antisense technology, continues to reinforce North America's leadership in the global market
U.S. Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The U.S. antisense oligonucleotide for neuromuscular disorders market captured the largest revenue share of 86% in 2024 within North America. This dominance is fueled by a well-established biotechnology ecosystem, early access to innovative therapies, and strategic initiatives by key players such as Sarepta Therapeutics and Ionis Pharmaceuticals. The U.S. has seen increasing adoption of antisense oligonucleotide drugs for treating genetic neuromuscular disorders, supported by orphan drug designations and strong payer support for advanced treatments. Government funding and patient advocacy initiatives are also contributing to market expansion.
Europe Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The Europe antisense oligonucleotide for neuromuscular disorders market is projected to expand at a substantial CAGR during the forecast period, propelled by growing investments in orphan drug development and strong clinical trial activity. Regulatory incentives provided by the European Medicines Agency (EMA), such as reduced fees and market exclusivity, are encouraging the development of antisense oligonucleotides for rare neuromuscular diseases. Patient awareness, combined with centralized healthcare systems, is driving early diagnoses and access to novel RNA-based therapies across key European countries.
U.K. Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The U.K. antisense oligonucleotide for neuromuscular disorders market is expected to grow at a noteworthy CAGR during the forecast period, driven by the country's focus on genomic medicine and rare disease research. The NHS Genomic Medicine Service and collaboration with organizations such as Genomics England are enhancing precision diagnosis and therapeutic adoption. In addition, favorable reimbursement policies and the UK’s active role in clinical trial networks make it a fertile environment for antisense oligonucleotide innovation.
Germany Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The Germany antisense oligonucleotide for neuromuscular disorders market is anticipated to expand at a considerable CAGR, backed by strong government funding for biotech research, the presence of top-tier academic institutions, and a high level of public healthcare expenditure. The country’s progressive approach to personalized medicine and participation in multi-center trials for antisense therapies further drive market growth. High adoption of advanced diagnostics supports the identification and treatment of neuromuscular disorders at earlier stages.
Asia-Pacific Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The Asia-Pacific antisense oligonucleotide for neuromuscular disorders market is projected to grow at the fastest CAGR of 20.4% from 2025 to 2032, owing to rapid advancements in healthcare infrastructure, government support for rare disease initiatives, and a growing patient population. Countries such as China, Japan, and South Korea are investing heavily in RNA-based therapies and expanding local manufacturing capacities. Rising awareness, coupled with increasing accessibility to genetic testing, is aiding early diagnosis and supporting therapy demand.
Japan Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The Japan antisense oligonucleotide for neuromuscular disorders market is gaining significant momentum due to its aging population, strong innovation in biotechnology, and government prioritization of rare diseases. Organizations such as AMED (Japan Agency for Medical Research and Development) are funding the development of antisense therapies. Japan’s fast regulatory reviews and partnerships between local pharma companies and global biotech firms are creating a conducive ecosystem for rapid market growth.
China Antisense Oligonucleotide for Neuromuscular Disorders Market Insight
The China antisense oligonucleotide for neuromuscular disorders market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by expanding healthcare coverage, a robust domestic pharma industry, and the inclusion of rare diseases in national health strategies. The country's commitment to becoming a global leader in biopharmaceutical innovation is fostering antisense oligonucleotide research and commercialization. In addition, local companies are forming alliances with international biotech players to introduce advanced RNA-based treatments across hospitals and specialized care centers.
Antisense Oligonucleotide for Neuromuscular Disorders Market Share
The antisense oligonucleotide for neuromuscular disorders industry is primarily led by well-established companies, including:
- Ionis Pharmaceuticals, Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Biogen Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Wave Life Sciences Ltd. (Singapore)
- Exicure, Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- Stoke Therapeutics, Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- VANDA PHARMACEUTICALS (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- PepGen Inc (U.S.)
- BioMarin (U.S.)
Latest Developments in Global Antisense Oligonucleotide for Neuromuscular Disorders Market
- In July 2025, Sarepta Therapeutics secured first-ever FDA Platform Technology Designation for its rAAVrh74 viral vector used in SRP‑9003 (bidridistrogene xeboparvovec), currently under Phase 3 trial for limb‑girdle muscular dystrophy type 2E/R4 (LGMD2E/R4). This designation is designed to streamline the development and regulatory process across multiple therapeutic programs using the same platform technology
- In May 2025, the Japanese regulatory authority granted conditional approval for Elevidys (Sarepta’s gene therapy for Duchenne muscular dystrophy) targeting children aged 3–8 years. This milestone sparked a 5.5% stock surge and unlocked milestone payments of up to $103.5 million
- In June–July 2025, the FDA announced an investigation into multiple fatalities tied to Sarepta’s gene therapy Elevidys. The company temporarily suspended shipments for non-ambulatory DMD patients and is evaluating an enhanced immunosuppressive protocol while preparing for a possible boxed warning label to address liver-related safety concerns
- In July 2025, Sarepta initiated a strategic restructuring, laying off about 500 employees (~36% of its workforce) and reallocating focus toward its siRNA platform, while maintaining support for its four FDA‑approved antisense drugs and ongoing trials
- In early 2025, promising Phase 1 results for salanersen, Biogen’s investigational once-yearly antisense oligonucleotide for spinal muscular atrophy (SMA), were reported—showing ~70% reduction in neurofilament light chain levels and functional improvements in motor milestones, paving the way for Phase 3 trials
- In 2025 preclinical research, a novel antisense oligonucleotide showed success in restoring dysferlin protein in cells from patients with dysferlinopathy via exon skipping, indicating expansion of ASO applications beyond DMD and SMA
- In January 2024, Vanda Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) had approved its Investigational New Drug (IND) application to study VCA-894A for the treatment of Charcot-Marie-Tooth disease, axonal, type 2S (CMT2S), in a patient. This condition is linked to cryptic splice site variants in the IGHMBP2 gene. VCA-894A is a novel antisense oligonucleotide (ASO) designed to specifically target these cryptic splice site variants within the immunoglobulin mu-binding protein 2 (IGHMBP2). Mutations in IGHMBP2 are believed to contribute significantly to the development of CMT2S, likely due to the degeneration of alpha motor neurons and subsequent deterioration of the peripheral nervous system.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.