Global Atopic Dermatitis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
17.05 Billion
USD
32.04 Billion
2024
2032
USD
17.05 Billion
USD
32.04 Billion
2024
2032
| 2025 –2032 | |
| USD 17.05 Billion | |
| USD 32.04 Billion | |
|
|
|
|
Atopic Dermatitis Treatment Market Size
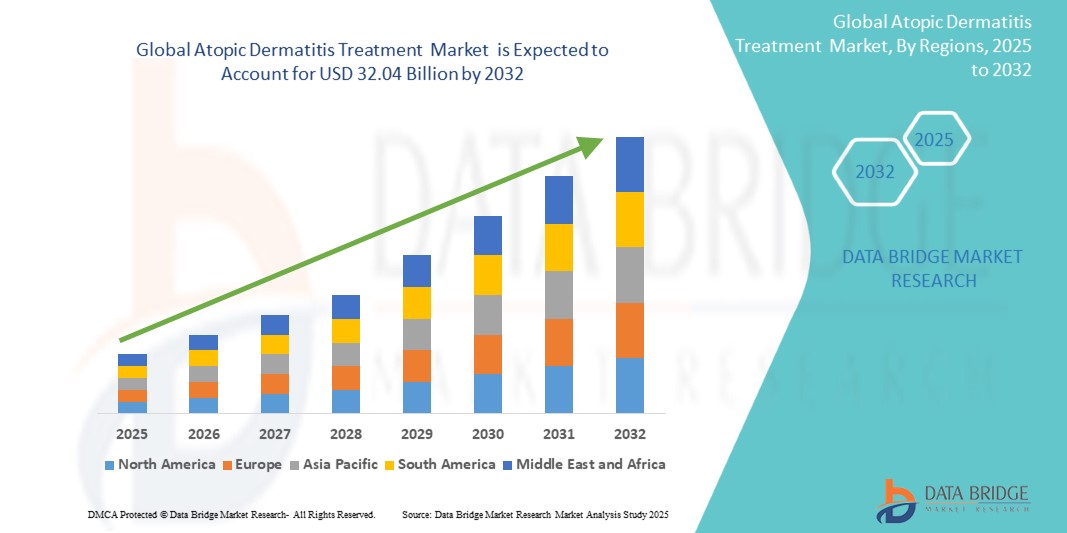
- The Global Atopic Dermatitis Treatment Market was valued at USD 17.05 Billion in 2024 and is expected to reach USD 32.04 Billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 8.2%, primarily driven by the anticipated launch of therapies
- This growth is driven by factors such as the Increasing Prevalence of Atopic Dermatitis.
Atopic Dermatitis Treatment Market Analysis
- Atopic dermatitis or atopic eczema treatment refers to a holistic approach to treat atopic dermatitis. The treatment for chronic skin diseases includes rehydrating the skin using emollients and topical steroids in order to reduce inflammation and itching of skin.
- The demand for atopic dermatitis treatments is primarily driven by the increasing prevalence of the condition and advancements in dermatological therapies. A significant portion of the market is fueled by the growing use of biologics and systemic treatments.
- The North America region stands out as one of the dominant regions for atopic dermatitis treatments, driven by its advanced healthcare infrastructure and high adoption of innovative therapies.
- For instance, the number of treatments for atopic dermatitis has steadily increased. In regions like North America, the demand for advanced therapies continues to grow, driving innovations in treatment protocols and expanding the use of biologics and other targeted therapies for managing this chronic skin condition.
- Globally, biologic therapies for atopic dermatitis rank as one of the most crucial treatments in dermatology clinics, following traditional corticosteroids, and play a pivotal role in ensuring precision and efficacy in managing this chronic skin condition, offering significant improvements in patients' quality of life.
Report Scope andAtopic Dermatitis Treatment Market Segmentation
|
Attributes |
Atopic Dermatitis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Atopic Dermatitis Treatment Market Trends
“Rising Adoption of Combination Therapies and Biomarker-Based Treatments in Atopic Dermatitis Management”
- One prominent trend in the Global Atopic Dermatitis Treatment Market is the increasing use of combination therapies and biomarker-based approaches to enhance treatment efficacy.
- Combining topical treatments, systemic therapies, or biologics has shown improved clinical outcomes in managing atopic dermatitis across different patient demographics.
- For instance, the combination of dupilumab with other topical treatments or systemic therapies has demonstrated superior efficacy in managing moderate to severe atopic dermatitis, leading to better patient outcomes and gaining regulatory approvals as a preferred treatment regimen.
- Biomarker-driven treatment strategies, such as targeting patients with high IL-13 or IL-4 expression, are enabling more precise and effective use of biologic therapies like dupilumab in atopic dermatitis.
- This trend is reshaping dermatology treatment protocols, driving innovation in clinical research, and expanding the market potential for targeted biologics through more personalized and effective therapeutic options.
Atopic Dermatitis Treatment Market Dynamics
Driver
“Increasing Prevalence of Atopic Dermatitis”
- The increasing prevalence of atopic dermatitis is a major market driver. This disease affects a large part of the world's population, increasing the demand for effective treatment options.
- As biologics have emerged as a transformative approach in dermatology, treatments like dupilumab play a central role in harnessing the body's immune system to manage atopic dermatitis more effectively.
- Atopic dermatitis treatments' expanding list of approved indications and favorable clinical outcomes have led to their widespread adoption as both monotherapies and part of combination treatments.
- The growing investment in dermatology research, coupled with increasing awareness and healthcare access in emerging markets, is further accelerating the drug’s uptake globally.
For instance,
- In recent years, regulatory agencies such as the U.S. FDA and the European Medicines Agency have approved various Atopic Dermatitis treatments for multiple indications, expanding its accessibility and usage in clinical practice.
- According to the World Health Organization (WHO), global cases of atopic dermatitis are expected to rise significantly, driving demand for advanced therapies like biologic treatments to address the growing burden.
- As the prevalence of atopic dermatitis rises and the healthcare industry shifts toward biologic treatments, the demand for advanced therapies continues to grow, reinforcing their role as key drivers in the global dermatology treatment landscape.
Opportunity
“Emerging Atopic Dermatitis Treatment Market”
- Expanding healthcare infrastructure in emerging markets provides a significant opportunity for the atopic dermatitis treatment market. As economic growth and healthcare costs increase in these regions, the demand for effective treatment options is expected to increase, creating a favorable market environment.
- AI-powered tools can analyze large datasets, including genetic profiles, biomarkers, and patient health records, to identify individuals who are most likely to benefit from Atopic Dermatitis treatments, supporting personalized medicine approaches.
- These technologies also aid in monitoring treatment response and disease progression in real-time, allowing for timely therapy adjustments and reducing adverse effects.
For instance,
- In 2024, research published in Nature Medicine highlighted the use of AI algorithms to predict immune-related adverse events in patients undergoing immune checkpoint inhibitor therapy, allowing clinicians to preemptively mitigate risks.
- The growing application of AI in oncology enhances clinical decision-making and facilitates more cost-effective and efficient healthcare delivery, opening new growth avenues for the Atopic Dermatitis Treatment market globally.
Restraint/Challenge
“High Cost Associated with Treatment”
- The high cost associated with the treatment of atopic dermatitis significantly limits the market. The costs of long-term care, including medications and wellness visits, can be burdensome for patients and limit their access to appropriate treatment options.
- Atopic dermatitis treatments, particularly immune checkpoint inhibitors, can cost tens of thousands of dollars annually per patient, creating a significant financial burden for healthcare systems and patients.
- The high treatment costs often limit access to advanced immunotherapies, especially in regions without strong reimbursement frameworks or universal healthcare coverage.
For instance,
- The high cost o fAtopic Dermatitis Treatment therapy poses a significant challenge for market penetration, particularly in low- and middle-income countries where healthcare budgets and insurance coverage are limited
- Treatment with immune checkpoint inhibitors such as Atopic Dermatitis Treatment can cost tens of thousands of dollars per patient annually, making it financially burdensome for healthcare systems and patients alike.
- This cost barrier often restricts access to advanced immunotherapies, especially in regions lacking robust reimbursement frameworks or universal healthcare coverage
Atopic Dermatitis Treatment Market Scope
The market is segmented on the basis of type, application, dosage, demographic, route of administration, end user and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Administration |
|
|
By End User |
|
|
By Distribution Channel |
|
Atopic Dermatitis Treatment Market Regional Analysis
“North America is the Dominant Region in the Atopic Dermatitis Treatment Market”
- North America leads the Global Atopic Dermatitis Treatment Market, due to the new product launches and early treatment adoption within the region.
- U.S. holds the largest market share due to its strong pipeline of oncology clinical trials, early regulatory approvals, and widespread usage of immunotherapy across multiple cancer indications
- Robust reimbursement frameworks, substantial investments in oncology research, and the presence of leading biopharmaceutical companies such as Bristol-Myers Squibb further bolster the region's market dominance
- In addition, high awareness among healthcare providers and patients regarding immuno-oncology treatments continues to drive demand for Atopic Dermatitis Treatment across various cancer care centers
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to witness the fastest growth during the forecast period because of the rise in healthcare expenditure in the region.
- Countries such as China and India are emerging as high-potential markets due to a growing patient population and government initiatives aimed at improving cancer care infrastructure
- Japan continues to play a significant role with its early adoption of immunotherapies, sophisticated healthcare system, and supportive regulatory environment for innovative drugs
- In China and India, the growing focus on affordable oncology care, increasing healthcare expenditure, and strategic partnerships between local and global pharmaceutical companies are accelerating market expansion for Atopic Dermatitis Treatment
Atopic Dermatitis Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Boehringer Ingelheim International GmbH (Germany)
- AbbVie Inc. (U.S.)
- Bayer AG (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Novartis AG (Switzerland)
- LEO Pharma A/S (Denmark)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- Pfizer Inc. (U.S.)
- GALDERMA LABORATORIES, L.P. (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Encore Dermatology, Inc. (U.S.)
- GSK plc (U.K.)
- AstraZeneca (U.K.)
- Bausch Health Companies Inc. (Canada)
Latest Developments in Global Atopic Dermatitis Treatment Market
- In September 2024, the U.S. FDA approved EBGLYSS (lebrikizumab-lbkz) developed by Eli Lilly and Company for the treatment of moderate to severe atopic dermatitis (eczema) adult and children aged 12 years and above who weigh at least 40kg that is not well manage by topical treatment. This approval is expected to boost market growth.
- In August 2023, Lynk Pharmaceuticals Co., Ltd. announced positive topline results from its Phase II clinical trial of LNK01001 for adults with atopic dermatitis (AD). After 12 weeks of treatment, preliminary data indicated that patients in both the low-dose and high-dose groups demonstrated significant improvements in Eczema Area and Severity Index (EASI) scores compared to the placebo group. The successful completion of clinical trial and approval of product is anticipated to drive market growth.
- In June 2022, the U.S. FDA approved Dupixent (dupilumab) developed by Sanofi for the treatment of moderate to severe atopic dermatitis (eczema) children aged 6 months to 5 years whose disease not controlled with treatment of topical drugs. This approval is anticipated to drive market growth.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.