Global Atypical Mycobacteriosis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.26 Billion
USD
3.06 Billion
2024
2032
USD
2.26 Billion
USD
3.06 Billion
2024
2032
| 2025 –2032 | |
| USD 2.26 Billion | |
| USD 3.06 Billion | |
|
|
|
|
Atypical Mycobacteriosis Treatment Market Size
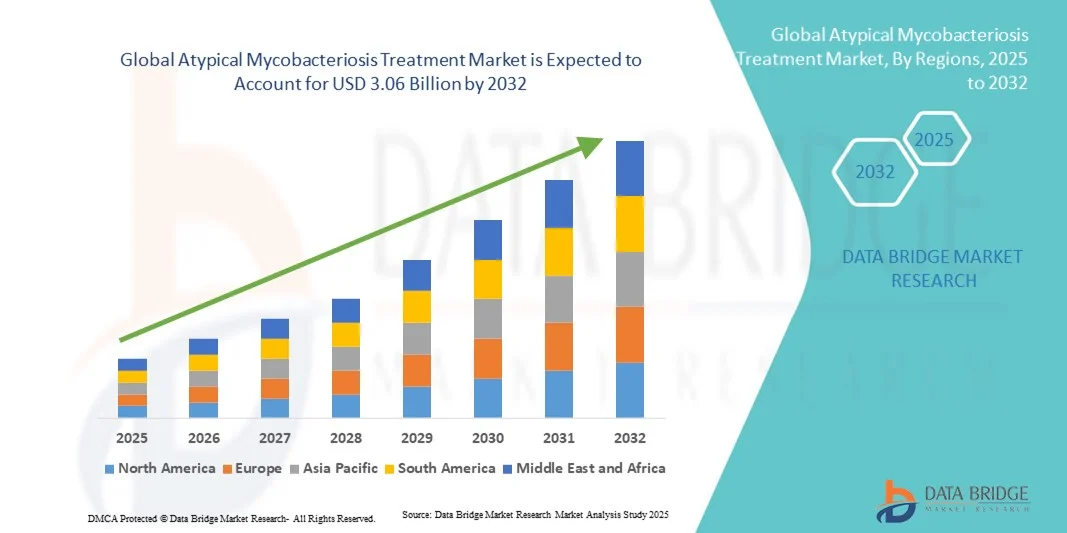
- The global atypical mycobacteriosis treatment market size was valued at USD 2.26 billion in 2024 and is expected to reach USD 3.06 billion by 2032, at a CAGR of3.90% during the forecast period
- The market growth is largely fueled by the increasing prevalence of atypical mycobacterial infections and advancements in diagnostic technologies, enabling earlier and more accurate detection of the disease across diverse patient populations
- Furthermore, rising demand for effective, targeted, and combination-based therapies is positioning atypical mycobacteriosis treatment as a critical focus area within infectious disease management. These converging factors are accelerating the adoption of novel treatment solutions, thereby significantly boosting the industry's growth
Atypical Mycobacteriosis Treatment Market Analysis
- Atypical Mycobacteriosis Treatment is becoming increasingly important in global healthcare systems due to rising incidence of nontuberculous mycobacterial infections, antibiotic resistance, and improved diagnostics that enable earlier detection and treatment
- The escalating demand for effective therapies is primarily fueled by the aging population, increasing prevalence of chronic lung diseases, growing number of immunocompromised patients, and advancements in antimicrobial drug development
- North America dominated the atypical mycobacteriosis treatment market with the largest revenue share of 45.0% in 2024, characterized by advanced healthcare infrastructure, higher awareness, and strong presence of pharmaceutical companies, with the U.S. experiencing substantial growth in treatment adoption and clinical trials for new antibiotics
- Asia-Pacific is expected to be the fastest growing region in the atypical mycobacteriosis treatment market during the forecast period, supported by increasing urbanization, rising healthcare expenditure, and greater access to diagnostic facilities
- The oral segment dominated the atypical mycobacteriosis treatment market with the largest revenue share of 62.4% in 2024, as oral therapy remains the cornerstone for most NTM treatment regimens
Report Scope and Atypical Mycobacteriosis Treatment Market Segmentation
|
Attributes |
Atypical Mycobacteriosis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Atypical Mycobacteriosis Treatment Market Trends
Enhanced Convenience Through AI and Precision Medicine
- A significant and accelerating trend in the global atypical mycobacteriosis treatment market is the growing integration of artificial intelligence (AI) and advanced precision medicine approaches into diagnostic and therapeutic processes. This convergence of technologies is improving early detection, enabling more personalized treatment plans, and enhancing patient monitoring outcomes
- For instance, in July 2023, Insilico Medicine announced the application of its AI-driven drug discovery platform to explore novel antimycobacterial agents targeting nontuberculous mycobacteria, aiming to accelerate the development of more effective therapies
- AI-based tools are increasingly being used to analyze complex genomic and clinical data, which allows physicians to tailor antibiotic regimens to specific NTM strains, predict resistance patterns, and reduce treatment failures. Moreover, precision medicine enables clinicians to design patient-specific therapeutic strategies, taking into account co-morbidities and individual tolerance levels
- The adoption of these intelligent, data-driven approaches is fundamentally reshaping clinical expectations by moving away from “one-size-fits-all” antibiotic regimens towards highly customized treatments. This transformation is expected to significantly improve patient compliance, reduce side effects, and enhance long-term cure rates
- Consequently, research institutions and pharmaceutical companies are increasingly investing in AI-powered diagnostic platforms and precision therapeutics, positioning themselves at the forefront of innovation in atypical mycobacteriosis care
- The demand for technologically advanced and patient-centered treatment models is growing rapidly across both developed and emerging markets, as healthcare systems aim to address rising infection rates and reduce the burden of prolonged therapy
Atypical Mycobacteriosis Treatment Market Dynamics
Driver
Growing Need Due to Rising Incidence of NTM Infections and Advancements in Diagnostics
- The increasing prevalence of nontuberculous mycobacterial (NTM) infections, coupled with improved access to advanced diagnostic technologies, is a significant driver for the heightened demand for atypical mycobacteriosis treatments
- For instance, in March 2024, Oxford Nanopore Technologies introduced an enhanced sequencing platform that enabled more rapid and accurate identification of mycobacterial species, allowing clinicians to initiate targeted therapy sooner and improve patient outcomes
- As more patients are being diagnosed due to better laboratory techniques, including molecular assays and next-generation sequencing, the demand for effective treatment regimens is accelerating. The rise in immunocompromised populations, including individuals with HIV/AIDS, transplant recipients, and elderly patients with chronic lung diseases, further fuels this demand
- Moreover, global health initiatives aimed at addressing antimicrobial resistance are prioritizing the development of new drugs and repurposing existing ones, which in turn drives market growth by expanding the therapeutic arsenal available to clinicians
- This trend towards earlier detection, coupled with rising infection rates, highlights the urgent need for effective, long-term management solutions, reinforcing the critical role of this market within the broader infectious disease treatment landscape
Restraint/Challenge
Concerns Regarding Drug Resistance and High Cost of Long-Term Therapy
- Concerns surrounding the growing resistance of mycobacterial strains to conventional antibiotics, along with the high cost of prolonged therapy, present a significant challenge to broader treatment adoption
- For instance, in October 2022, a study published in The Lancet Microbe reported that certain strains of Mycobacterium abscessus showed resistance to commonly prescribed macrolide antibiotics, leading to higher treatment failure rates and increased healthcare costs
- Drug resistance not only complicates treatment protocols but also extends therapy duration, often requiring patients to undergo multi-drug regimens lasting 12 to 24 months, which can be both physically and financially burdensome
- High treatment costs pose a major barrier, especially in developing regions, where limited healthcare budgets and lower patient affordability hinder access to effective care. Even in developed nations, the financial strain of long-term antibiotic use and associated hospitalizations can be considerable
- Overcoming these challenges requires a two-fold approach: investment in the development of new antimycobacterial agents and supportive therapies, as well as policy-driven measures to subsidize treatment costs and improve patient adherence. Without addressing these barriers, achieving sustained market growth and improved public health outcomes will remain difficult
Atypical Mycobacteriosis Treatment Market Scope
- By Drug Type
On the basis of drug type, the atypical mycobacteriosis treatment market is segmented into antituberculous drugs, clarithromycin, aminoglycosides, quinolones, and others. The antituberculous drugs segment dominated the market with the largest revenue share of 41.5% in 2024, owing to their well-established role as the backbone of treatment regimens for nontuberculous mycobacterial infections. Drugs such as rifampin, isoniazid, and ethambutol are widely prescribed due to their proven efficacy, broad clinical acceptance, and inclusion in international treatment guidelines. These agents are often combined with macrolides like clarithromycin to improve therapeutic outcomes and reduce relapse rates. Their dominance is reinforced by the availability of generic versions, which enhances affordability and accessibility, particularly in emerging markets. Rising prevalence of chronic pulmonary conditions, immunocompromised patients, and the elderly population has further driven demand. Hospitals and clinics rely heavily on these drugs for both initial and maintenance therapy, with outpatient oral regimens facilitating long-term management. Established safety profiles, predictable pharmacokinetics, and clinician familiarity make antituberculous drugs the first-choice option in most regions. Moreover, ongoing clinical trials and post-marketing studies continue to support their continued use in combination therapies. Patient adherence is further improved by fixed-dose combinations and simplified dosing schedules. Overall, the antituberculous drugs segment remains the most trusted and widely adopted therapeutic category globally.
The fastest-growing drug type is the “Others” category, projected to expand at a CAGR of 7.9% from 2025 to 2032. This segment includes novel antimycobacterial agents, repurposed antibiotics, and investigational compounds targeting drug-resistant NTM strains. Growth is driven by the urgent need to address rising antimicrobial resistance and treatment failures in refractory cases. For example, experimental compounds identified through AI-driven drug discovery platforms are showing promise in early-stage clinical studies. Pharmaceutical companies are investing in combination therapies that incorporate these new agents with standard regimens to enhance efficacy and reduce treatment duration. Regulatory incentives and faster approval pathways for orphan and rare infection treatments further accelerate growth. The growing awareness among physicians and patients about treatment optimization and resistance management is contributing to higher uptake of these innovative drugs. Patient-centric approaches, such as personalized dosing and monitoring, are further reinforcing its growth trajectory.
- By Route of Administration
On the basis of route of administration, the atypical mycobacteriosis treatment market is segmented into oral, parenteral, and others. The oral segment held the largest revenue share of 62.4% in 2024, as oral therapy remains the cornerstone for most NTM treatment regimens. Oral antibiotics like clarithromycin, rifampin, and ethambutol enable long-term outpatient treatment, which is essential given the prolonged duration of therapy often required for NTM infections. The convenience of oral administration enhances patient adherence, reduces hospital visits, and lowers overall treatment costs. This route is preferred for initial therapy, maintenance, and combination regimens in both adults and pediatric populations. Global availability of generic oral formulations further ensures affordability and accessibility. Physicians widely recommend oral therapy for stable patients with moderate disease severity, while maintaining flexibility to escalate to parenteral treatment if needed. Oral therapy also supports the integration of multidisciplinary care, enabling pharmacists and nurses to provide adherence support and counseling. Patient education on adherence, monitoring for side effects, and the use of fixed-dose combinations strengthens its position as the most widely adopted route.
The parenteral segment is projected to witness the fastest CAGR of 8.2% from 2025 to 2032, driven by its critical role in severe, drug-resistant, or disseminated NTM infections. Parenteral therapy, primarily with aminoglycosides like amikacin or intravenous macrolides, allows for rapid attainment of therapeutic drug levels, essential in refractory cases. Hospitals and specialty clinics increasingly adopt IV protocols for patients who cannot tolerate oral medications or require combination therapy to overcome resistance. The segment growth is supported by rising incidence of multidrug-resistant NTM infections, expanding hospital infrastructure, and the availability of novel IV formulations. Patient monitoring during parenteral therapy ensures safety, reduces complications, and improves clinical outcomes. In addition, pharmaceutical innovation in long-acting or once-daily IV formulations enhances convenience and adherence. Specialized infusion centers and outpatient parenteral antimicrobial therapy (OPAT) programs further facilitate the adoption of parenteral treatments. The segment benefits from research in optimizing dosing schedules, minimizing nephrotoxicity, and integrating parenteral therapy with oral maintenance therapy for long-term efficacy. Rising awareness among clinicians about aggressive early intervention strategies for resistant NTM cases is also contributing to robust growth in this segment.
- By End-Users
On the basis of end-users, the atypical mycobacteriosis treatment market is segmented into hospitals, clinics, and others. The hospitals segment dominated with a revenue share of 54.8% in 2024, as most patients with NTM infections require initial inpatient care, complex diagnostics, and management of long-term antibiotic regimens. Hospitals provide the infrastructure for parenteral therapy, monitoring adverse effects, and performing susceptibility testing. Multidisciplinary care teams, including infectious disease specialists, pulmonologists, and pharmacists, ensure personalized treatment and adherence support. Hospitals also serve as primary sites for clinical trials and introduction of new therapies. Education on long-term therapy, dose adjustments, and monitoring protocols are integrated within hospital settings to improve patient outcomes. Chronic pulmonary patients, immunocompromised individuals, and post-transplant patients are typically managed in hospitals for the initial phases of therapy. In addition, hospitals maintain structured pharmacy and laboratory services to monitor therapy efficacy. The growing incidence of severe NTM infections globally reinforces hospitals’ critical role. Regulatory approvals and healthcare policies often channel high-value drugs through hospitals, enhancing revenue share.
The clinics segment is expected to register the fastest growth at a CAGR of 7.5% from 2025 to 2032. The expansion of outpatient services is a key factor driving this growth, as clinics increasingly cater to patients who require ongoing monitoring but do not need hospitalization. Rising awareness among physicians and patients about atypical mycobacteriosis, early diagnosis, and timely treatment has further strengthened the role of clinics in the healthcare ecosystem. Clinics are now offering structured oral therapy programs, where patients can receive prescriptions, counseling, and adherence support in a convenient setting. Home-based care programs are being integrated with clinic services, allowing patients to continue long-term antibiotic therapy under remote supervision. Clinics also provide follow-up visits, routine laboratory testing, and side-effect management, which ensures continuity of care after hospital discharge.
- By Distribution Channel
On the basis of distribution channel, the atypical mycobacteriosis treatment market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment held the largest share of 48.6% in 2024, driven by its direct integration with patient treatment programs, provision of parenteral therapies, and availability of high-cost specialty antibiotics. Hospital pharmacies ensure timely supply of medications, adherence monitoring, and patient counseling. Complex regimens and multi-drug combinations are easier to manage in hospital pharmacy settings, enhancing treatment outcomes. Hospitals dispense first-line and combination therapies, and pharmacists collaborate with clinicians for dose optimization, side-effect management, and drug interactions. Integration with electronic medical records enables tracking, inventory management, and coordination of therapy. Hospitals also provide access to newer or investigational drugs through clinical programs, supporting patient care. In addition, hospital pharmacies facilitate long-term therapy through outpatient extensions and monitoring programs. High trust in hospital pharmacy distribution ensures reliability and patient confidence. Expansion of hospital networks in emerging markets supports ongoing growth and dominance of this segment.
The online pharmacy segment is projected to grow at the fastest CAGR of 9.1% from 2025 to 2032. The increasing trend of e-commerce is a major factor driving this growth, as more patients turn to digital platforms for purchasing medications conveniently from home. Rising patient preference for home delivery of chronic medications has strengthened the adoption of online pharmacies, particularly among individuals undergoing long-term treatment for atypical mycobacteriosis. Digital adherence programs offered by online platforms help patients track their medication schedules, receive reminders, and maintain treatment compliance, which is crucial for therapies that often span several months. Growing accessibility in both urban and semi-urban regions is expanding the reach of online pharmacies to previously underserved populations. Online pharmacies also offer discreet packaging and delivery, which appeals to patients concerned with privacy during prolonged antibiotic therapy. Integration with telemedicine services allows for prescription verification, online consultation, and patient counseling, further enhancing the user experience.
Atypical Mycobacteriosis Treatment Market Regional Analysis
- North America dominated the atypical mycobacteriosis treatment market with the largest revenue share of 45.0% in 2024, driven by advanced healthcare infrastructure, higher awareness of atypical mycobacterial infections, and the strong presence of leading pharmaceutical companies. The region benefits from well-established hospitals, specialized clinics, and widespread access to diagnostic facilities
- High adoption of novel antibiotic regimens, clinical research programs, and active participation in global clinical trials further strengthen market dominance. The presence of robust healthcare systems ensures timely diagnosis, structured treatment protocols, and continuous patient monitoring, which collectively improve treatment outcomes. Rising prevalence of chronic pulmonary conditions and immunocompromised populations also drives the need for effective therapies
- North America continues to serve as a hub for R&D, encouraging the development and adoption of innovative combination therapies. Patient awareness programs, insurance coverage for specialized treatments, and advanced hospital infrastructure support the rapid uptake of antibiotics. Multidisciplinary approaches, including infectious disease specialists and pharmacists, enhance adherence and optimize clinical results. Overall, North America remains the most mature and revenue-generating market for atypical mycobacteriosis treatment globally
U.S. Atypical Mycobacteriosis Treatment Market Insight
The U.S. atypical mycobacteriosis treatment market captured the largest revenue share within North America in 2024, fueled by widespread adoption of new antibiotics, participation in clinical trials, and growing awareness among physicians and patients. Hospitals and specialty clinics are increasingly implementing advanced treatment protocols, including combination therapies for drug-resistant NTM infections. The U.S. benefits from high-quality healthcare infrastructure, access to cutting-edge diagnostics, and extensive R&D capabilities in both public and private sectors. Rising incidence of chronic lung diseases, immunocompromised populations, and post-transplant patients contributes to demand for effective therapies. Ongoing clinical trials targeting novel antibiotics and regimens further drive market growth. The availability of generic and branded drugs ensures treatment accessibility, while insurance coverage facilitates patient adherence. Physicians are increasingly adopting patient-centered approaches, integrating monitoring, counseling, and follow-up programs to optimize outcomes. The U.S. market is also supported by government initiatives, public health campaigns, and collaboration with pharmaceutical companies to accelerate the development of new therapies. Growing research on drug-resistant strains and expanded hospital networks continue to reinforce the U.S.’s leading position within North America.
Europe Atypical Mycobacteriosis Treatment Market Insight
The Europe atypical mycobacteriosis treatment market is projected to expand at a substantial CAGR throughout the forecast period, driven by stringent healthcare regulations, the rising prevalence of NTM infections, and increasing adoption of advanced treatment protocols. Improved access to diagnostic facilities and standardized hospital and clinic practices support early detection and effective management. European countries such as Germany and the U.K. show steady growth due to strong healthcare infrastructure, clinical research initiatives, and patient awareness programs. Government policies and insurance coverage further encourage treatment uptake.
U.K. Atypical Mycobacteriosis Treatment Market Insight
The U.K. atypical mycobacteriosis treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing adoption of evidence-based treatment protocols and the rising incidence of atypical mycobacterial infections. Hospitals and clinics are implementing advanced diagnostic and therapeutic approaches, while government awareness campaigns and a robust pharmaceutical industry support patient access to effective antibiotics. The focus on early diagnosis and chronic disease management reinforces market growth.
Germany Atypical Mycobacteriosis Treatment Market Insight
The Germany atypical mycobacteriosis treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by heightened awareness of atypical mycobacterial infections and the demand for advanced therapeutic options. Germany’s emphasis on healthcare innovation, well-established infrastructure, and sustainable treatment protocols drives adoption across both hospitals and clinics. Integration of diagnostic advancements, antibiotic stewardship programs, and access to clinical trials enhances patient management.
Asia-Pacific Atypical Mycobacteriosis Treatment Market Insight
The Asia-Pacific atypical mycobacteriosis treatment market is poised to grow at the fastest CAGR during the forecast period, supported by increasing urbanization, rising healthcare expenditure, and greater access to diagnostic facilities. Countries such as China, Japan, and India are witnessing rapid adoption of treatment protocols due to increasing prevalence of NTM infections and improved healthcare infrastructure. Expansion of specialized hospitals and clinics, combined with availability of novel antibiotic regimens, drives market growth. Government initiatives, insurance coverage, and patient awareness programs further support treatment adoption. Affordable therapies and domestic pharmaceutical manufacturing make treatments more accessible to a broader population, reinforcing Asia-Pacific’s position as the fastest-growing region.
Japan Atypical Mycobacteriosis Treatment Market Insight
The Japanese atypical mycobacteriosis treatment market is gaining momentum due to the country’s advanced healthcare system, high awareness of NTM infections, and growing elderly population. Hospitals and clinics are integrating innovative treatment protocols with advanced diagnostics, ensuring effective patient management. The adoption of combination antibiotic therapies and home-based monitoring programs is increasing, supporting market expansion.
China Atypical Mycobacteriosis Treatment Market Insight
The China atypical mycobacteriosis treatment market accounted for the largest revenue share in Asia-Pacific in 2024, driven by rapid urbanization, a growing middle-class population, and high adoption of healthcare innovations. Both hospitals and clinics are expanding access to effective antibiotic regimens. Government initiatives promoting public health, increased healthcare expenditure, and improved diagnostic infrastructure are key factors propelling market growth. Domestic pharmaceutical manufacturing and availability of affordable therapies further support widespread adoption of treatment protocols.
Atypical Mycobacteriosis Treatment Market Share
The Atypical Mycobacteriosis Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Johnson & Johnson and its affiliates (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Merck & Co. Inc. (U.S.)
- Sanofi S.A. (France)
- GSK plc (GSK) (U.K.)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Astellas Pharma Inc. (Japan)
- Cipla Limited (India)
- Paratek Therapeutics, Inc. (U.S.)
- Insmed Incorporated (U.S.)
- Sandoz (Switzerland)
- Sun Pharmaceutical Industries Ltd. (India)
- Zydus Pharmaceuticals, Inc. (India)
Latest Developments in Global Atypical Mycobacteriosis Treatment Market
- In May 2025, scientists introduced a novel engineered drug candidate, VOMG, demonstrating efficacy against Mycobacterium abscessus, a challenging non-tuberculous mycobacterium (NTM). VOMG inhibits cell division and shows broad-spectrum activity against other microbial pathogens. The development candidate is set to advance to Investigational New Drug (IND)-enabling studies to support clinical development
- In April 2025, researchers reported the successful discovery of second-generation rifamycins with improved efficacy against mycobacterial infections. These next-generation rifamycins are poised to enter IND-enabling studies, aiming to enhance treatment options for NTM infections
- In March 2025, clinical trials for bacteriophage therapy targeting Mycobacterium abscessus, a prevalent NTM pathogen, were initiated. This innovative approach aims to address antibiotic resistance and provide alternative treatment strategies for NTM lung disease
- In February 2025, clinical trials commenced to evaluate the efficacy and safety of Clofazimine Inhalation Suspension (MNKD-101) in treating NTM lung disease. The ICoN-1 Phase 3 study compares this treatment against a placebo when added to guideline-based therapy, potentially offering a new therapeutic option for patients
- In January 2025, the U.S. Food and Drug Administration (FDA) approved Arikayce (amikacin liposome inhalation suspension) as part of a combination antibacterial regimen for treating refractory Mycobacterium avium complex (MAC) lung disease. This approval provides a new treatment avenue for patients with chronic lung infections
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.