Global Autonomic Dysreflexia Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.12 Billion
USD
5.08 Billion
2024
2032
USD
3.12 Billion
USD
5.08 Billion
2024
2032
| 2025 –2032 | |
| USD 3.12 Billion | |
| USD 5.08 Billion | |
|
|
|
|
Autonomic Dysreflexia Treatment Market Size
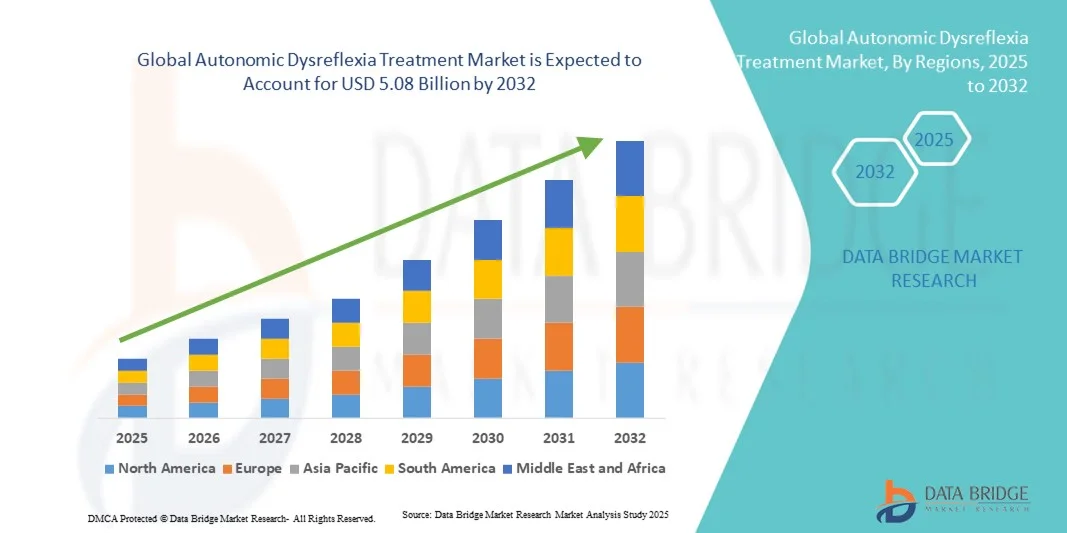
- The global autonomic dysreflexia treatment market size was valued at USD 3.12 billion in 2024 and is expected to reach USD 5.08 billion by 2032, at a CAGR of 6.30% during the forecast period
- The market growth is largely fueled by the increasing prevalence of spinal cord injuries, growing awareness of autonomic dysreflexia as a serious medical emergency, and advancements in diagnostic and therapeutic approaches, which are collectively driving demand for effective treatment solutions
- Furthermore, the rising focus on patient-centric care, expanding clinical research into neurogenic disorders, and the introduction of improved pharmacological and non-pharmacological interventions are accelerating the adoption of Autonomic Dysreflexia Treatment solutions, thereby significantly boosting the industry's growth
Autonomic Dysreflexia Treatment Market Analysis
- Autonomic Dysreflexia Treatment, encompassing both pharmacological and non-pharmacological interventions, is becoming increasingly essential in managing patients with spinal cord injuries due to its role in preventing life-threatening hypertensive episodes and improving overall patient quality of life
- The rising demand for Autonomic Dysreflexia Treatment is primarily fueled by increasing awareness among healthcare professionals, growing spinal cord injury cases worldwide, and expanding access to advanced neurorehabilitation facilities
- North America dominated the autonomic dysreflexia treatment market with the largest revenue share of 35% in 2024, supported by advanced healthcare infrastructure, early adoption of innovative neurotherapeutic approaches, and the strong presence of key pharmaceutical and medical device companies. The U.S. continues to lead the region due to increased clinical research on spinal cord injuries and the availability of specialized treatment centers
- Asia-Pacific is expected to be the fastest-growing region in the autonomic dysreflexia treatment market during the forecast period, projected to register a CAGR of 23.8%, driven by rising healthcare expenditure, increasing awareness of spinal cord-related disorders, and improving access to neurological care in countries such as China, Japan, and India
- The Oral route dominated the market with 64.8% share in 2024, attributed to its convenience, cost-effectiveness, and widespread patient acceptance
Report Scope and Autonomic Dysreflexia Treatment Market Segmentation
|
Attributes |
Autonomic Dysreflexia Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Autonomic Dysreflexia Treatment Market Trends
Advancements in Emergency Management and Pharmacological Interventions
- A significant and accelerating trend in the global autonomic dysreflexia (AD) treatment market is the increasing development of advanced pharmacological and non-pharmacological approaches for rapid and sustained management of hypertensive crises in patients with spinal cord injuries
- The market is witnessing greater focus on precision diagnosis and early recognition tools, supported by advancements in telemonitoring and continuous blood pressure tracking to prevent severe complications such as intracerebral hemorrhage and cardiac arrest
- Recent years have seen growing clinical adoption of short-acting antihypertensive agents such as nifedipine, captopril, and nitroglycerin formulations for acute management, coupled with ongoing trials assessing novel neuroprotective drugs and autonomic modulators to prevent recurrence
- Furthermore, hospitals and rehabilitation centers are increasingly integrating clinical decision-support algorithms to identify and treat AD episodes more rapidly, ensuring adherence to updated clinical guidelines by organizations such as the American Spinal Injury Association
- Non-invasive monitoring systems and mobile alert platforms for remote supervision of spinal injury patients are emerging as vital tools for early detection of dysreflexic episodes, reducing hospitalizations and improving quality of life
- This growing clinical and technological convergence aimed at improving patient safety, minimizing secondary complications, and enhancing post-injury care protocols is driving innovation and reshaping the landscape of autonomic dysreflexia management globally
Autonomic Dysreflexia Treatment Market Dynamics
Driver
Rising Prevalence of Spinal Cord Injuries and Increasing Awareness of AD Management
- The global rise in spinal cord injury (SCI) cases — estimated at over 250,000–500,000 new cases annually according to WHO — has significantly driven the demand for improved AD treatment options
- Autonomic dysreflexia, a life-threatening hypertensive episode primarily seen in patients with injuries above the T6 level, is now better recognized as a critical emergency requiring immediate and standardized intervention
- Increasing awareness among healthcare professionals about early identification and management of AD has led to stronger implementation of clinical protocols, improving diagnosis and survival rates
- Pharmaceutical advances, including more targeted vasodilators and short-acting antihypertensives, have contributed to effective control of acute episodes and reduced morbidity in patients
- For instance, ongoing development of combination therapy protocols and integrated care pathways in tertiary centers worldwide has led to improved outcomes and faster recovery
- In addition, the expansion of rehabilitation and spinal injury centers globally — particularly in North America, Europe, and parts of Asia-Pacific — is facilitating easier access to specialized treatment, boosting market growth for pharmacologic and supportive therapies
- Overall, the growing focus on patient education, continuous monitoring, and guideline-based treatment protocols is propelling the global Autonomic Dysreflexia Treatment market, making it a critical area within neurorehabilitation and emergency medicine
Restraint/Challenge
Limited Awareness in Developing Regions and High Cost of Specialized Care
- Despite growing research and clinical progress, limited awareness and delayed recognition of autonomic dysreflexia in developing countries remain significant challenges to effective management
- Many patients and even primary care providers in low- and middle-income regions lack familiarity with the symptoms and emergency management of AD, leading to underdiagnosis and poor outcomes
- The high cost associated with long-term management of spinal cord injuries — including pharmacologic therapy, emergency care, and rehabilitation services — also limits patient access to quality treatment, particularly in resource-constrained healthcare systems
- Furthermore, the shortage of trained neurologists and physiatrists, as well as inadequate infrastructure for continuous monitoring and acute care, hampers the delivery of timely interventions
- Pharmaceutical development in this field faces additional constraints due to the rarity of the condition and small patient populations, which discourage large-scale R&D investments
- Addressing these challenges through government-funded awareness programs, patient education initiatives, and increased funding for rare neurological disorders will be essential to expand access and foster sustained market growth
- Overall, while advancements in clinical guidelines and pharmacological management are promising, overcoming disparities in diagnosis and affordability remains crucial for global progress in autonomic dysreflexia treatment
Autonomic Dysreflexia Treatment Market Scope
The market is segmented on the basis of injury type, drug type, route of administration, and distribution channel.
- By Injury Type
On the basis of injury type, the Autonomic Dysreflexia Treatment market is segmented into Complete Spinal Cord Injury and Incomplete Spinal Cord Injury. The Complete Spinal Cord Injury segment dominated the market revenue share with 58.7% in 2024, owing to the high prevalence of severe spinal cord injuries leading to loss of sensory and motor functions below the injury site. Patients with complete injuries are at a significantly higher risk of developing autonomic dysreflexia, driving sustained demand for therapeutic interventions. The need for continuous medical management, hospitalization, and pharmaceutical support further reinforces segment dominance. Moreover, growing advancements in emergency and acute care have improved survival rates among patients with complete spinal cord injuries, indirectly contributing to the expanding treatment pool for autonomic dysreflexia. Increasing clinical awareness and established diagnostic protocols also favor the segment’s prominence across both developed and emerging healthcare markets.
The Incomplete Spinal Cord Injury segment is expected to witness the fastest growth, registering a CAGR of 8.9% from 2025 to 2032. This growth is attributed to rising early-detection rates, improved neurorehabilitation facilities, and advancements in minimally invasive surgical techniques. Patients with partial neurological preservation are more likely to experience recurring dysreflexic episodes, requiring long-term management through pharmacological and non-pharmacological interventions. Enhanced research efforts targeting neuroprotective recovery and regenerative therapies are expanding the treatment scope for incomplete spinal injuries. Furthermore, the increase in traumatic accidents, sports-related injuries, and geriatric falls continues to drive demand for this category globally.
- By Drug Type
On the basis of drug type, the Autonomic Dysreflexia Treatment market is segmented into Corticosteroids, Muscle Relaxants and Anti-spastic Drugs, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Anti-Depressants, Anticonvulsants, and Others. The Muscle Relaxants and Anti-spastic Drugs segment held the largest revenue share of 41.3% in 2024, driven by their efficacy in managing hyperreflexia and muscle spasticity associated with autonomic dysreflexia. Drugs such as baclofen and tizanidine remain the standard of care for reducing involuntary muscle contractions and stabilizing autonomic responses. Increasing physician preference for oral and intrathecal formulations further supports segment leadership. Moreover, continuous research into next-generation spasmolytics and combination therapies enhances the long-term therapeutic potential of this category. Rising patient adherence due to improved tolerability profiles also bolsters the market performance of muscle relaxants and anti-spastic agents.
The Anticonvulsants segment is projected to grow at the fastest rate, recording a CAGR of 9.4% from 2025 to 2032, owing to their emerging use in managing neuropathic pain and autonomic instability in dysreflexic patients. Drugs such as gabapentin and pregabalin are increasingly prescribed to regulate nerve signaling and prevent sympathetic overactivity. Expanding clinical research into neuroprotective and anti-inflammatory benefits of anticonvulsants further accelerates growth. In addition, the introduction of novel formulations with extended release properties and minimal side effects enhances patient compliance. Growing off-label adoption across rehabilitation centers and specialized spinal injury units is expected to strengthen the anticonvulsant segment’s position in the coming years.
- By Route of Administration
On the basis of route of administration, the Autonomic Dysreflexia Treatment market is segmented into Oral and Intravenous. The Oral route dominated the market with 64.8% share in 2024, attributed to its convenience, cost-effectiveness, and widespread patient acceptance. Oral medications enable self-administration and long-term maintenance therapy, which is crucial for patients requiring chronic management. The availability of multiple drug classes—including muscle relaxants, anticonvulsants, and NSAIDs—in oral dosage forms ensures broad accessibility across various healthcare settings. Pharmaceutical advancements in extended-release tablets and improved bioavailability further enhance therapeutic outcomes. In addition, the increasing preference among clinicians for outpatient treatment regimens has significantly boosted the adoption of oral administration routes worldwide.
The Intravenous (IV) segment is anticipated to expand at the fastest CAGR of 8.6% from 2025 to 2032, driven by the growing need for rapid symptom control in acute autonomic dysreflexia episodes. IV formulations are critical in emergency and hospital-based care, particularly for severe hypertension and autonomic crises. The rising prevalence of inpatient treatment cases in trauma centers and neurorehabilitation hospitals supports segment growth. The continuous development of fast-acting and safer IV drug formulations with precise dosage control also contributes to this expansion. Increasing healthcare expenditure and the establishment of specialized spinal cord injury units globally are further strengthening the demand for IV administration.
- By Distribution Channel
On the basis of distribution channel, the Autonomic Dysreflexia Treatment market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. The Hospital Pharmacies segment accounted for the largest market revenue share of 55.9% in 2024, owing to the high dependence on institutional supply chains for acute care and critical treatment management. Hospital pharmacies serve as the primary channel for dispensing intravenous medications and specialized prescriptions that require medical supervision. The rising number of spinal cord injury admissions and expanding neuro-intensive care units worldwide drive strong sales through this channel. Furthermore, partnerships between hospitals and pharmaceutical companies for the direct supply of critical care drugs enhance accessibility and ensure quality control.
The Online Pharmacies segment is projected to witness the fastest growth, registering a CAGR of 10.1% from 2025 to 2032. Increasing digitalization of healthcare services, growing awareness of telemedicine, and convenience in obtaining long-term prescriptions for chronic dysreflexia management contribute to its growth. Patients with limited mobility due to spinal injuries increasingly prefer home delivery of medications, fueling online sales. In addition, the rising number of government initiatives promoting e-pharmacy regulations and the integration of AI-driven medication management platforms are accelerating adoption. Expanding e-commerce infrastructure and improved drug authentication processes further enhance consumer trust, positioning online pharmacies as a rapidly emerging distribution channel.
Autonomic Dysreflexia Treatment Market Regional Analysis
- North America dominated the autonomic dysreflexia treatment market with the largest revenue share of 38.9% in 2024
- Attributed to advanced healthcare infrastructure, early adoption of innovative neurological treatments, and the strong presence of leading pharmaceutical and biotechnology companies
- The region benefits from high awareness levels among healthcare professionals, well-established reimbursement systems, and ongoing clinical research on spinal cord injuries, which collectively enhance treatment accessibility and outcomes
U.S. Autonomic Dysreflexia Treatment Market Insight
The U.S. autonomic dysreflexia treatment market captured the largest revenue share in 2024 within North America. This dominance is driven by significant government and private sector investments in rare neurological disorder research, robust patient support networks, and increasing clinical trials focused on spinal cord injury-related complications. The growing adoption of advanced pharmacological therapies, coupled with early diagnosis rates and availability of minimally invasive treatment options, continues to strengthen the U.S. market position.
Europe Autonomic Dysreflexia Treatment Market Insight
The Europe autonomic dysreflexia treatment market is projected to expand at a notable CAGR during the forecast period, supported by rising healthcare expenditures, a growing geriatric population, and enhanced diagnostic awareness. European nations are increasingly implementing advanced neurorehabilitation therapies and pharmacological protocols for managing autonomic dysreflexia. In addition, strong collaboration among research institutions and medical organizations fosters the development of novel drug combinations and treatment regimens, particularly in countries such as Germany, France, and the U.K.
U.K. Autonomic Dysreflexia Treatment Market Insight
The U.K. autonomic dysreflexia treatment market is expected to grow at a considerable CAGR through 2032, driven by an increasing prevalence of spinal cord injuries and proactive government initiatives to support rare disease research. The country’s focus on advanced clinical infrastructure and the adoption of digital healthcare platforms are improving diagnosis and management outcomes. Furthermore, strategic partnerships between academic centers and pharmaceutical firms are fostering innovation in both oral and intravenous treatment formulations.
Germany Autonomic Dysreflexia Treatment Market Insight
The Germany autonomic dysreflexia treatment market is expected to register steady growth during the forecast period, owing to the nation’s emphasis on advanced neurology care and cutting-edge treatment facilities. Germany’s strong biopharmaceutical base and healthcare funding mechanisms are accelerating access to corticosteroids, anti-spastic drugs, and other critical medications used in autonomic dysreflexia management. In addition, increasing hospital-based clinical research is driving the evolution of personalized treatment options.
Asia-Pacific Autonomic Dysreflexia Treatment Market Insight
The Asia-Pacific autonomic dysreflexia treatment market is projected to grow at the fastest CAGR of 23.8% during 2025–2032, fueled by increasing spinal cord injury cases, expanding healthcare access, and government efforts to enhance awareness about rare neurological disorders. Emerging economies such as China, Japan, and India are witnessing rapid advancements in diagnostic imaging and neurorehabilitation technologies. Pharmaceutical companies in the region are also investing in cost-effective formulations and expanding their distribution networks through hospital and retail pharmacies.
Japan Autonomic Dysreflexia Treatment Market Insight
The Japan autonomic dysreflexia treatment market is experiencing steady growth, supported by the country’s advanced medical infrastructure, growing elderly population, and rising demand for neurological care. Continuous research in neuroprotective and muscle relaxant therapies, combined with increasing clinical trial participation, is driving innovation in treatment protocols. Japan’s focus on quality care and accessibility is expected to maintain its leadership within the regional market.
China Autonomic Dysreflexia Treatment Market Insight
The China autonomic dysreflexia treatment market accounted for the largest revenue share in the Asia-Pacific region in 2024, attributed to expanding healthcare infrastructure, rising healthcare expenditure, and government-backed initiatives for rare disease management. Increasing awareness among healthcare providers, along with domestic pharmaceutical innovation and enhanced drug distribution through online and hospital pharmacies, is further boosting market growth in China.
Autonomic Dysreflexia Treatment Market Share
The Autonomic Dysreflexia Treatment industry is primarily led by well-established companies, including:
- AbbVie Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- GSK plc (U.K.)
- AstraZeneca plc (U.K.)
- Roche Holding AG (Switzerland)
- Lilly (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bayer AG (Germany)
- Merck & Co., Inc. (U.S.)
- Sanofi (France)
- Boehringer Ingelheim International GmbH (Germany)
- Takeda Pharmaceutical Company Limited (Japan)
- Biogen Inc. (U.S.)
- Mallinckrodt Pharmaceuticals (Ireland)
- Amneal Pharmaceuticals LLC (U.S.)
- Cipla Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
Latest Developments in Global Autonomic Dysreflexia Treatment Market
- In May 2022, a Japanese clinical study reported the successful use of transcutaneous spinal cord stimulation (tSCS) in patients with high-thoracic spinal cord injury that markedly reduced the incidence of autonomic dysreflexia episodes. The study demonstrated that repeated tSCS sessions improved autonomic cardiovascular regulation, lowered resting and reactive blood pressure spasms, and enhanced patients’ quality of life by reducing dysreflexic triggers. This represents one of the earliest systematic applications of neuromodulation specifically targeting autonomic dysreflexia management
- In March 2023, a Phase II clinical trial (NCT05024487) began enrolling individuals with spinal cord injury at or above T6 to test a novel therapeutic protocol aimed at prevention of complications due to autonomic dysreflexia, involving continuous cardiovascular monitoring and tailored antihypertensive therapy. The trial seeks to quantify reductions in hypertensive crisis incidence, stroke risk, and hospital admissions among participants, marking a shift toward proactive rather than purely reactive dysreflexia management
- In November 2023, research published in the Journal of Neurophysiology revealed that 22% of all parameter test sessions during implanted spinal cord stimulation for motor recovery triggered asymptomatic autonomic dysreflexia events in patients with spinal cord injury. The finding underscores the importance of vigilant cardiovascular monitoring when using neuromodulation therapies and influences device-manufacturers and clinicians to integrate dysreflexia risk mitigation protocols in spinal stimulation programs
- In March 2025, the NSW Agency for Clinical Innovation (Australia) released an updated referenced clinical guideline titled “Treatment of Autonomic Dysreflexia for Adults and Adolescents with Spinal Cord Injuries”. The guideline includes a revised algorithm for acute and preventive management of dysreflexia episodes, highlights expanded roles for outpatient monitoring, introduces newer antihypertensive drug combinations, and addresses long-term follow-up protocols. It aims to standardize care across specialist and non-specialist units globally
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.